Tribal health officials work to fill vaccination gaps as measles outbreak spreads
How did your country report this? Share your view in the comments.
Diverging Reports Breakdown
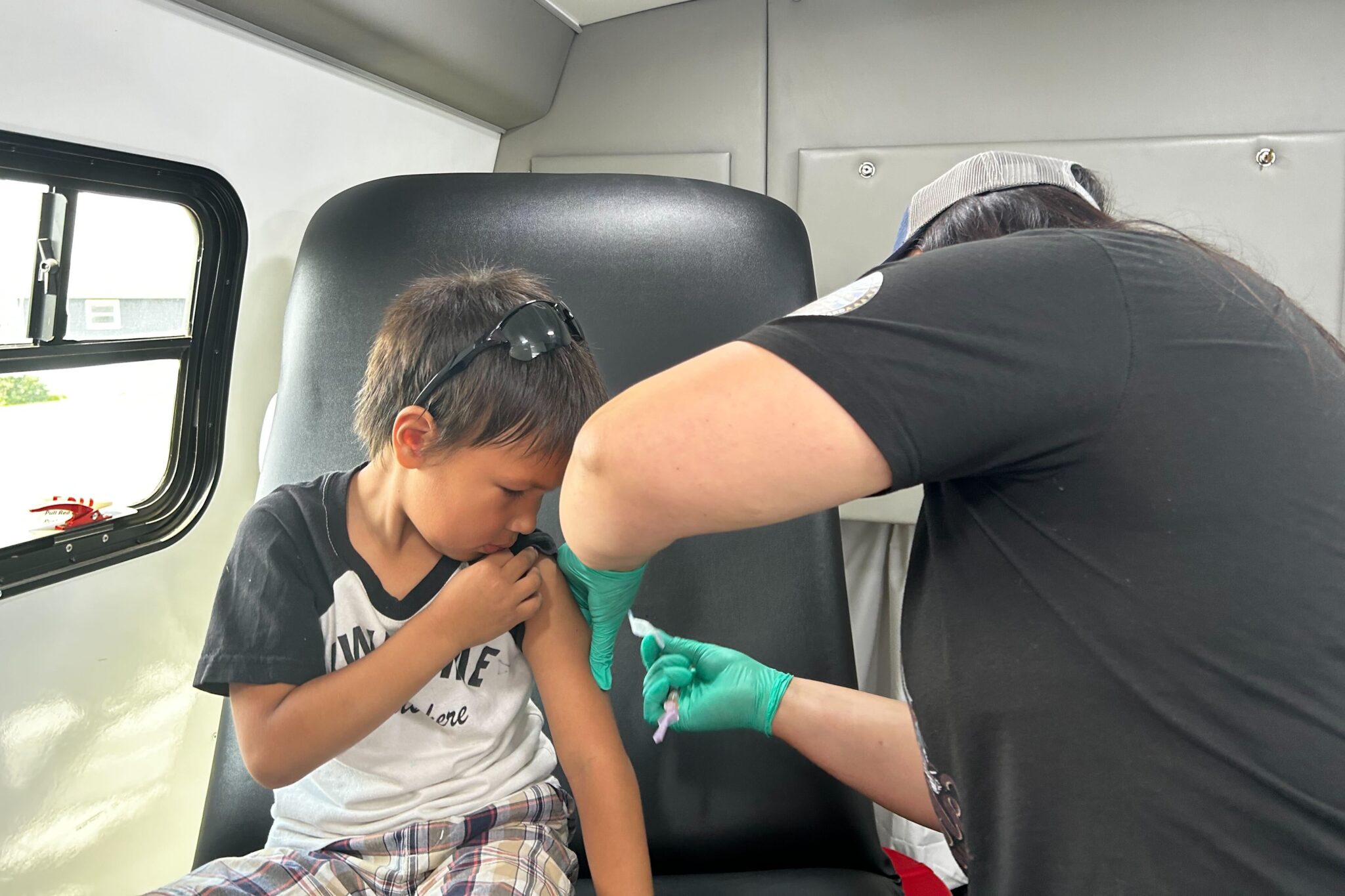
South Dakota’s Dept. of Health sends out mobile vans to help vaccinate against measles
The “Wellness on Wheels” clinics offer various services, including vaccinations, STD testing, and prenatal care. The fleet boasts five vehicles equipped to provide immunizations, test for sexually transmitted diseases such as syphilis. The effort fills in gaps to public health care access across the state, especially in rural and tribal communities. Counties with the fewest kindergarteners vaccinated per capita for measles, mumps and rubella include Faulk, Jones and Hutchinson, state data shows. The state Department of Health recently reported the state’s first case this year in Meade County in western South Dakota, and a second case was reported last week in Rapid City. The department plans to have staff drive the buses to rural communities more regularly to increase exposure and encourage use of the clinics, the health department secretary says.. More than a hundred rural hospitals in the U.S. have closed in the last decade. The program, launched in April of last year, cost about $800,000 in federal funding.
South Dakota Searchlight
AI-assisted summary South Dakota plans to deploy mobile health clinics to underserved areas in response to measles outbreaks.
Two measles cases were recently reported in Meade County and Rapid City, prompting health officials to urge monitoring for symptoms.
The “Wellness on Wheels” clinics offer various services, including vaccinations, STD testing, and prenatal care.
Funded by COVID relief funds, the mobile clinics aim to bridge healthcare gaps in rural and tribal communities.
The program seeks to increase accessibility by bringing healthcare directly to communities, similar to the historical practice of doctor’s home visits.
The South Dakota Department of Health plans to send its fledgling mobile clinics to underserved and undervaccinated areas of the state in response to the nationwide measles outbreak making its way to the state.
Last year, South Dakota reported its first measles case in nine years.
The state Department of Health recently reported the state’s first case this year in Meade County in western South Dakota. Last week, on Friday, a second case was reported in Rapid City. People who visited Sam’s Club in that city on June 1, or Dakota Premier Medical Center the following day were urged by the department to monitor themselves for symptoms for 21 days.
Measles is a highly contagious viral disease that spreads through the air. Those who lack immunity from vaccination or past infection are highly likely to catch it from an infected person.
As surrounding states report more cases, Health Department Secretary Melissa Magstadt said the state’s “Wellness on Wheels” clinics can help encourage vaccinations.
The fleet boasts five vehicles equipped to provide immunizations, test for sexually transmitted diseases such as syphilis, as well as provide screenings, prenatal care and other support. The effort fills in gaps to public health care access across the state, especially in rural and tribal communities, Magstadt said.
“It’s about how we can actively use these tools to reach underserved populations,” Magstadt said. “It’s not something I would have thought about looking to leverage for something like measles vaccinations before.”
The department hasn’t decided where to send their fleet. Counties with the fewest kindergarteners vaccinated per capita for measles, mumps and rubella include Faulk, Jones and Hutchinson, state data shows.
South Dakota counties that share tribal land and rural counties in south-central areas of the state rank the worst for clinical care use and access in the state, according to the University of Wisconsin Population Health Institute’s 2023 report.
Federal COVID relief funds paid for Wellness on Wheels.
“Because of the pandemic, public health infrastructure was found to be wanting,” Magstadt said. That infrastructure missed “critical pieces” that hadn’t been invested in, she said, such as health care access in rural areas. More than a hundred rural hospitals in the U.S. have closed in the last decade.
The program, launched in April of last year, cost about $800,000 in federal funding. The state’s public health COVID funding was also used to support a community health worker program, update emergency medical service equipment and telemedicine access, analyze the state of emergency medical services in South Dakota, and build a Public Health Lab and department training center.
Magstadt said staff working with the Women, Infants and Children (WIC) program requested the mobile units. WIC is a federal-state program that provides healthy food, nutrition education and health care referrals to low-income women and their young children.
So far, Wellness on Wheels staff have mainly driven to events. Magstadt said the department has focused on increasing awareness of the program and building trust in communities and among tribal leaders. She plans to have staff drive the buses to rural communities more regularly to increase exposure and encourage use.
“Being consistently at a facility or place every other week will help people find it,” Magstadt said. “We talk about the importance of STI testing, for example, but if you don’t know where to get tested then that’s another barrier.”
Magstadt plans to have the department park one of the vehicles at a homeless shelter in Rapid City this summer as well to encourage underserved urban communities to seek services.
“We like people to be connected to primary care services, but there are unique situations where it’s harder to get to health care facilities,” Magstadt said.
She compared the mobile clinics as a return to home visits by doctors. That practice largely ceased in the 1960s due to cost efficiencies.
“It’s a part of this menu of health care access and options no longer requiring people to come to a clinic or health care system,” Magstadt said, “but health care being taken to patients and families who need it.”
South Dakota Searchlight is part of States Newsroom, the nation’s largest state-focused nonprofit news organization.
REPORT: The Biden-Harris Administration Roadmap for Pandemic Preparedness and Response
President Biden came into office facing the worst public health crisis in more than a century. In the first year of the pandemic, nearly 400,000 Americans died of COVID-19. The Biden-Harris Administration worked hand-in-hand with doctors, nurses, businesses, unions, community organizations, governors, mayors, and citizens to put vaccines at the center of the United States’ response. The Administration stood up the largest free vaccination program in American history: mobilizing 90,000 vaccination locations; standing up mass vaccination sites with the ability to administer more than 125,000 shots a day; deploying over 9,000 federal personnel to support vaccinations nationwide – including over 5,000 active-duty troops, and launching vaccinefinder.org to provide current information on locations for vaccination. As a result of these efforts, over 270 million people received at least one shot of a CO VID-19 vaccine by May 2023. A December 2022 analysis from the Commonwealth Fund suggested that COVID.-19 vaccinations saved over 3 million American lives and successfully prevented over 18 million hospitalizations.
Even before taking office, President-elect Biden recognized that the U.S. needed an emergency response that was worthy of the crisis it faced – a response that would leave no stone unturned and that would leverage the full force of the federal government, the innovation of the private sector, and the determination of the American people. Building on decades of research and planning efforts, President Biden, on his first full day in office, released the first comprehensive National Strategy for the COVID-19 Response and Pandemic Preparedness. This strategy focused on building a response to this virus that would give people the tools and transparent communication they needed to protect themselves, reopen our schools, and get our economy moving again.
The following report outlines the numerous actions the Biden-Harris Administration took to combat COVID-19 both nationally and globally, and it serves as a roadmap for how the U.S. can effectively respond to pandemics and public health threats in the future. In addition to this public-facing report, this Administration is leaving behind a three-step playbook that future Administrations can use to continue to protect the nation and effectively respond to any future biological threat.
1 – Taking Immediate Action to Respond to the COVID-19 Pandemic
Standing Up the Largest Vaccination Program in Our Country’s History
In President Biden’s first year of office, the Biden-Harris Administration worked hand-in-hand with doctors, nurses, businesses, unions, community organizations, governors, mayors, and citizens across every state, Tribe, and territory to put vaccines at the center of the United States’ COVID-19 response. These vaccines still remain the best tools available to lower the risk of hospitalization and death.
The Administration stood up the largest free vaccination program in American history: mobilizing 90,000 vaccination locations; standing up mass vaccination sites with the ability to administer more than a combined 125,000 shots a day; deploying over 9,000 federal personnel to support vaccinations nationwide – including over 5,000 active-duty troops, and launching vaccinefinder.org to provide current information on locations for vaccination. Another part of the federal government’s strategy to ensure access to COVID-19 vaccines for the American public was the Federal Retail Pharmacy Program (FRPP) for COVID-19 Vaccination. Pharmacies are readily accessible in the majority of communities in the U.S. – with most Americans living within five miles of a pharmacy. Recognizing this, the federal government made pharmacies a key part of its COVID-19 vaccination strategy, partnering with 21 retail and long-term care pharmacies to vaccinate Americans in more than 41,000 locations nationwide, including long-term care pharmacies.
As a result of these efforts, over 270 million people received at least one shot of a COVID-19 vaccine by May 2023. Additionally, a December 2022 analysis from the Commonwealth Fund suggested that COVID-19 vaccinations saved over 3 million American lives and successfully prevented over 18 million hospitalizations.
Increasing the Country’s Testing Supply
Under the leadership of the Biden-Harris Administration, America’s testing supply increased substantially, allowing Americans to quickly get answers without having to go to a doctor’s office, and to make informed decisions about their day-to-day activities. Less than a month after taking office, the Administration announced a $650 million investment to expand COVID-19 testing for schools and underserved populations, as well as an $815 million investment to increase domestic manufacturing of testing supplies so that we would have a more reliable supply when needed. The Administration, through HHS, also partnered with the private sector to develop and scale manufacturing of tests suitable for home use. Free testing sites were available at 21,500 locations around the country. This was made possible by federal action to expand pharmacy testing sites, a federal surge in free testing sites, delivery of tests to thousands of community health centers and rural health clinics, and $10 billion in American Rescue Plan (ARP) funding to provide tests to K-12 school districts. The Administration also invested nearly $6 billion in ARP funding to cover free testing for uninsured individuals, and to support testing in correctional facilities, shelters for people experiencing homelessness, and mental health facilities. To reach people experiencing homelessness, the U.S. Housing and Urban Development (HUD) collaborated with the Department of Health and Human Services (HHS) to provide tests across major U.S. cities.
The Biden Administration also stood up COVIDtests.gov through which Americans could order tests that were sent by the United States Postal Service directly to their homes — for free. By the end of the Public Health Emergency in May 2023, the Administration had distributed more than 750 million free COVID-19 tests, shipped directly to more than 85 million households. The Administration had also coordinated more than 50 million diagnostic tests in-person at pharmacy and community-based sites.
Collectively, these actions gave Americans the opportunity to keep both themselves and their communities safe, while getting back to school, work, and time with family and friends. Additionally, the Lancet Public Health journal recently published a study showing that making diagnostic tests available quickly during the COVID-19 pandemic mitigated an estimated 7 million hospitalizations and saved approximately 1.4 million lives.
Increasing Treatment Options for Americans
The Biden-Harris Administration also increased investment in the development, manufacturing, and procurement of COVID-19 treatments, which helped to minimize the severity of COVID-19 infections. By March 2022, about 5 million antiviral treatment courses were available to Americans, and the President announced the Test-to-Treat initiative to help make it easier for people at high risk of severe disease and those with limited financial means to quickly access free oral antiviral treatments. By April 2022, the U.S. government purchased 20 million treatment courses—more than any other country in the world and took action to nearly double the number of locations where Americans could get oral antivirals. The Administration also provided medical providers with more guidance, education and tools to help them understand and prescribe these treatments, and to help them inform the choices that the American people made about receiving safe and effective treatments.
2 – Ensuring an Equitable Pandemic Response and Recovery
On his first day in office, understanding that the pandemic had exacerbated severe and pervasive health and social inequities in America, President Biden issued an Executive Order on Ensuring an Equitable Pandemic Response and Recovery – which included the establishment of the COVID-19 Health Equity Task Force. From the start, the Administration took action to empower communities to improve access for all Americans to tests, therapeutics and vaccines.
In addition, the Administration supported partners through an all-of-society effort that increased response and recovery initiatives in support of communities in every corner of the country. In some communities, local chambers of commerce worked with business leaders to encourage flexibilities such as paid time off for their employees who needed to travel to a vaccination or testing center. In other communities, due to the Administration’s efforts, child care providers offered drop-in services for caregivers to get vaccinated. Some public transit authorities and ride-sharing companies provided free rides to vaccination sites, while churches, civic organizations, barbershops, and beauty salons opened their doors to be trusted spaces for testing or for vaccinations.
Ten months into the Biden-Harris Administration’s term, deaths had declined nearly 90% in Black, brown, and Indigenous communities; the gap in vaccination rates between Black and Latino/Hispanic adults and white adults had closed; and nearly 100% of schools were open for in-person instruction.
Investing in the Hardest-hit and Highest-risk communities:
The Biden-Harris Administration invested over $785 million from President Biden’s American Rescue Plan to support organizations that were building vaccine confidence across communities which historically had lower vaccination levels, including communities of color, rural populations, and low-income populations. The Administration bolstered the efforts of Tribal communities seeking to increase awareness of options to mitigate the spread of the virus, and it expanded public health systems’ ability to respond to the needs of older adults who have been among the highest risk for infection or death from COVID-19.
Additionally, recognizing that the pandemic had tremendous impacts on disabled individuals and resulted in new members of the disability community, the Administration prioritized Long COVID services, supports, and research in the context of disability; established a call line dedicated to ensuring individuals with disabilities can equitably utilize the Administration’s at-home test distribution program; ensured disabled individuals and other high-risk individuals could access at-home testing; and invested American Rescue Plan (ARP) resources to build COVID-19 vaccine confidence and access among people with disabilities.
Putting Community Health Centers at the Forefront of the Response:
Community Health Centers played a vital role in the Administration’s efforts to ensure an equitable response, as they served as the single largest source of comprehensive primary health care for medically underserved urban and rural communities. Because of the Administration’s efforts, these centers tested millions of patients for COVID-19, distributed millions of vaccine doses, increased access to telehealth in order to improve and expand patient care, and offered treatment options such as oral antiviral medications and monoclonal antibody therapy. Additionally, through its COVID-19 Testing Supply and COVID-19 N95 Mask Programs, the Administration enabled health centers to distribute millions of N95 masks, COVID-19 at-home test kits, and COVID-19 point-of-care testing supplies, at no charge to their patients and community members.
Supporting Community-Based Organizations in Vaccine Outreach to High-Risk Communities:
Through community-based organization vaccine outreach, the Administration was able to focus on empowering local trusted messengers and providing educational materials that served the most vulnerable populations. The Administration translated materials into 14 languages, and these were used by community- and faith-based organizations around the country, as well as by doctors’ offices, pharmacies, health centers, employers, and other groups. These education and outreach efforts allowed the Administration to reach the unvaccinated, deploy information about the importance of boosters, support pediatric vaccination efforts, and provide other important COVID-19 updates through trusted community members.
Building the Workforce to Support Underserved Communities:
President Biden’s American Rescue Plan provided a total of over $1.1 billion for community health, outreach, and health education workers—the largest ever one-time investment in the nation’s community health workforce. In the fall of 2022, the Administration invested $225 million in American Rescue Plan funds to train over 13,000 Community Health Workers (CHWs) – responding to the acute need to expand the health care workforce and address pandemic-related burnout. This effort supported apprenticeship programs for workers at over 500 health care and public health sites nationally, including emergency departments, community health centers, state and local public health departments, mobile health clinics, shelters, housing programs, faith-based organizations, and other locations where high-risk populations access care and receive services. The Administration also rapidly deployed over 14,000 community outreach workers through over 150 national and local organizations to deepen COVID-19 vaccine confidence, increase vaccination rates, and serve as trusted messengers in underserved communities. These actions built upon the efforts of the roughly 50,000 CHWs who were already working in American communities before the pandemic.
3- Getting America Back on its Feet
Countless lives were saved by the Administration’s efforts to ensure all Americans had access to safe tests, treatments and vaccines. In addition, robust support to employers minimized the impact of the COVID-19 pandemic. Thanks to these efforts, families nationwide were able to get back to work and school and the country’s economy recovered faster and more broadly than any of the other leading economies in the world.
Progress By the Numbers
In May 2023, compared to January 2021, COVID-19 deaths had declined by 95% and hospitalizations were down nearly 91% in the U.S.; those who were not vaccinated were more likely to be hospitalized or to die of COVID-19, compared to people who were vaccinated.
With the largest domestic vaccination program in history, the U.S. made it possible for over 270 million people to receive at least one shot of a COVID-19 vaccine by May 2023. At its peak, the Biden Administration COVID-19 vaccination program administered over 4 million vaccines in one day, or over 2,700 vaccines a minute, into the arms of the American people. Lifesaving treatments were widely available and used, with more than 15 million courses administered.
Through COVIDTests.gov, the Administration has delivered more than 921 million free COVID-19 tests – shipped directly to more than 85 million households – as of January 2025.
Through the Administration’s efforts, more than 50 million diagnostics tests were administered in-person at pharmacy and community-based sites.
As a result of the Biden-Harris Administration’s efforts, the economic recovery from the pandemic in the U.S. was historic. The American Rescue Plan (ARP) accelerated that economic recovery throughout 2021 and made it more resilient to challenges: one analysis found that the ARP resulted in 4 million more jobs and nearly doubled GDP growth – and that without it, the United States would have come close to a double-digit recession in spring 2021. The results of the ARP have also been historically equitable, with major progress against child poverty, food insecurity, and unemployment for low-income communities and communities of color.
Additionally, the Biden-Harris Administration’s COVID-19 response ensured that schools could reopen and families could get back to work. By the end of March 2020, all public schools in the United States were closed to slow the spread of the novel coronavirus SARS-CoV-2. In November 2020, 19 percent of districts remained fully remote, with 45 percent using hybrid models and 36 percent fully in person. Shortly after the start of the Biden-Harris Administration, in early May, 2021, just over 3 months after taking office, only 1 percent of districts across the country were fully remote, and over half of schools were fully in person.
These changes are reflected in the public’s perception of the pandemic’s impact on their lives. According to Gallup public opinion polling, in December 2020, 3/5th of Americans believed that COVID-19 in the U.S. was getting worse. By June 2021, that percentage had fallen to three percent of Americans. Additionally, over half of Americans worried about catching COVID in December 2020, and that number fell to less than 20% by June 2021.
Today, although much progress has been made, the Administration continues to ensure that Americans have what they need to stay safe, including by continuing to provide free COVID-19 tests through COVIDtests.gov. In addition, the Administration has extended the authorities which allow pharmacists and pharmacy technicians to continue to administer vaccines, allowing other healthcare workers to focus on other tasks that only they can perform. And, the Administration’s $5 billion investment in Project NextGen continues to accelerate and streamline the rapid development of the next generation of coronavirus vaccines and treatments through public-private collaborations.
In addition to addressing the immediate impact of COVID-19 infections, the Biden-Harris Administration recognized that millions of Americans continue to experience symptoms for months and sometimes years after their acute COVID-19 infection. To help better understand why this occurs and to develop potential treatments, the Biden-Harris Administration has dedicated billions of dollars to research efforts, developed the first-ever National Research Action Plan on Long COVID, and created an Advisory Committee on Long COVID.
4 – Ensuring the World Responded and Recovered from COVID-19
While the Biden-Harris Administration implemented all of these programs to help Americans fight COVID-19 here at home, the Administration also recognized that helping the rest of world quickly and effectively respond to the pandemic was critical to both our domestic and the broader global recovery. The United States committed to bringing the same urgency to international response and recovery efforts that we demonstrated domestically. On day one, President Biden called on his National Security Advisor to advance global health security, international pandemic preparedness, and global health resilience to support the global response to the COVID-19 pandemic. This included re-establishing the National Security Council’s team focused on health security and biodefense.
Restoring Partnerships with Critical, Life-saving Institutions: As soon as President Biden entered office, he ensured that the U.S. reversed its decision to withdraw from the World Health Organization – which was essential to coordinating a global response during the pandemic. In early 2021, United States committed $4 billion to support COVAX, the multilateral effort that aimed to accelerate the development and manufacturing of COVID-19 vaccines and to support equitable access for every country in the world. In two years, the United States provided over $16 billion to vaccinate the world, save lives, and build stronger health security. The United States also convened world leaders at two Global COVID-19 Summits, accelerating response efforts and mobilizing $3.2 billion in commitments to vaccinate the world, save lives, and build stronger health security.
Vaccinating the World: The United States donated more COVID-19 vaccines than any other country, and it was the first country to announce a purchase of vaccine doses solely for donation to other countries. The U.S. was also the first country to ensure the African Union could start receiving up to 110 million doses of Moderna at a reduced rate negotiated by the United States – and it was the first country to negotiate a deal to send vaccines directly to humanitarian settings and conflict zones to vaccinate displaced persons. Between May 2021 and February 2024, the Biden-Harris Administration donated – in partnership with COVAX, Caricom, the African Vaccine Acquisition Trust (AVAT), and bilaterally – nearly 700 million doses of COVID-19 vaccines to countries around the world. This included over 44 countries and economies in Sub-Saharan Africa, 31 countries in the Western Hemisphere, and 26 countries in Southern, Central, and Eastern Asia.
The COVID-19 pandemic also led to a dropoff in routine childhood immunization in many countries around the world, as they surged scarce resources to pandemic response. As a result, we began to see the reemergence of vaccine-preventable diseases, from measles to polio. In 2024, the United States Government pledged $1.58 billion to support Gavi, The Vaccine Alliance, over the next five years. This commitment builds on a 24-year partnership that has immunized over a billion children and saved 17 million lives. The new funding aims to vaccinate the next billion children within a decade, saving over eight million lives by reaching unvaccinated children, expanding vaccinations for diseases like malaria and cervical cancer, and enhancing emergency health preparedness. The United States, through Gavi, also supports the launch of the African Vaccine Manufacturing Accelerator, which will help African countries produce vaccines locally, promoting vaccine equity and swift responses to future health crises. In addition, the United States supports the Coalition for Epidemic Preparedness Innovations (CEPI), which is working to accelerate the development of life-saving vaccines against emerging disease threats, and to transform capability for rapid countermeasure development in response to future threats. Notable achievements include: the FDA approval of the world’s first Chikungunya vaccine and technology transfer to regional producers for regional supply to LMICs; the advancement through clinical development of vaccine candidates against Lassa, Nipah, and coronaviruses, among others; and the launch of a new Disease X Vaccine Library with six viral families prioritized as high risk.
Delivering Life-Saving Resources: In addition, the U.S. government delivered life-saving resources like oxygen, treatments, PPE, and other essential supplies worth more than $1 billion to countries experiencing outbreaks by March 2022. This included countries that were most affected by the pandemic. As an example, as India battled a devastating wave of the Delta variant, the United States delivered supplies worth more than $100 million to provide urgent relief. This included 15 million N95 masks, 1 million rapid diagnostic tests, and vaccine manufacturing supplies to help India make over 20 million doses of COVID-19 vaccines. In addition, the U.S. consistently provided immediate support to allies such as Brazil that were seeing disproportionate cases and deaths due to the pandemic – through providing much-needed ventilators, vaccines, personal protective equipment, and support for struggling businesses and communities.
Providing Technical Assistance and Supporting Vaccine Manufacturing: U.S. public health experts across multiple federal agencies worked side-by-side with on-the-ground providers – providing technical assistance in vaccine program implementation, care provision, and outbreak investigation. The United States respects countries’ right to protect public health and to promote access to medicines for all. Toward that end, the United States endorsed negotiations of a temporary waiver of WTO intellectual property rules to support access to COVID vaccines.
In addition, the U.S. increased the world’s capacity to manufacture vaccines and fostered an enabling environment for innovation, including by spurring African manufacturing. For example, the U.S. International Development Finance Corporation (DFC) provided a $3.3 million technical assistance grant and a follow-on $15 million loan to Institute Pasteur de Dakar (IPD) in Senegal to expand flexible vaccine manufacturing capacity for both routine and outbreak vaccines. IPD also received support from other U.S. government agencies on regulatory strengthening, workforce development and training, and research and development.
5) Managing Current Public Health Threats
The tools and strategies that the Biden-Harris Administration developed in response to the COVID-19 pandemic are applicable to a range of biological threats, including avian flu, Marburg, Ebola, mpox, COVID-19 variants, and others.
As an example, the National Wastewater Surveillance System has allowed the U.S. to glean more specific information on where avian flu is found in the environment, often before the first human or animal case has been confirmed. Additionally, Traveler-based Genomic Surveillance, which was among the first to detect multiple Omicron variants up to six weeks before they were reported elsewhere in the United States, continues to screen for other threats including new COVID-19 variants. Hospital data reporting also provides granular information on which hospitals may see strain due to admissions from COVID-19, Flu, and RSV each respiratory season.
Avian Flu: Protecting Human and Animal Health
Avian flu, or Influenza A(H5N1), was first detected in dairy cattle in the U.S. in late March 2024. While we have seen this virus in birds for decades and the risk to the general public remains low, the Administration immediately knew that the spread to cows and other mammals demanded serious attention and action. Within twenty-four hours of confirmation of the first case, interagency coordination groups began meeting at the senior leader and technical levels to synchronize support to State and local public health and agriculture officials. Since then, the interagency has worked with government, industry and other partners to ensure we keep communities healthy, safe, and informed – by monitoring and stopping transmission, keeping animals healthy, ensuring that our Nation’s food supply remains safe, and safeguarding the livelihood and well-being of American farmers and farmworkers. In total, since USDA began supporting state-led efforts to mitigate the risk of avian flu in poultry in 2022, the Biden-Harris administration has dedicated nearly $2.8 billion to this important work.
Monitoring the Virus and Stopping Transmission: Within a few weeks of the outbreak, USDA took action to stop the spread of the virus, issuing a federal order in April 2024 that mandated avian flu testing of all lactating dairy cattle moving between states. USDA also stood up a voluntary program for states and farmers to test their herds, implement biosecurity and created incentives for them to do so. By the end of 2024, USDA and its partner laboratories had run over 110,000 tests on dairy cattle and made more than 1,000 staff deployments to support response and traceback efforts on the ground, including 221 personnel currently deployed. In October 2024, USDA announced a nationwide milk testing initiative, requiring states to comprehensively monitor and respond to the presence of the virus in America’s dairies. Today, 28 states – representing nearly two-thirds of America’s dairy production – have joined this program. The remaining states are working to stand up the necessary infrastructure.
CDC has also been closely tracking the virus through a collaborative effort between CDC and many partners, including state, local, and territorial health departments; public health and clinical laboratories; clinics; and emergency departments. These include systems to monitor case reporting, laboratory monitoring at both public health and clinical labs, emergency department visits, hospitalizations, and assessing wastewater. These systems build on developments over the last four years as a result of the COVID-19 pandemic. Altogether, they provide us with early warning signs on where the virus is spreading, as well as visibility on whether there is any severe disease from avian flu. When human cases have been reported, CDC has engaged and supported state and local health officials with technical support, including the deployment of experts to the field to support public health investigations.
Since the start of the outbreak, USDA and CDC have been monitoring virus specimens using the latest techniques, to inform our response. When new human cases are reported, CDC’s national laboratory confirms the findings and performs timely genomic sequencing and other analysis to monitor for any concerning changes in the virus, as well as any potential impacts on our treatments and vaccines. This information has been released in technical reports and the sequences are made available on public servers. Similarly on the animal side, genetic sequences from this outbreak are shared by USDA, with over 4,500 raw or curated sequences having been posted to GISAID (the Global Initiative on Sharing Avian Influenza Data) or the National Center for Biotechnology Information (NCBI) Sequence Read Archive. USDA continually monitors these sequences for any potentially concerning changes and immediately shares any such findings with CDC.
Protecting Workers and the Public: Learning from bottlenecks and shortages in the very early COVID-19 response, the Administration has spent the last several years refilling our Strategic National Stockpile to ensure that we have the PPE, antivirals, tests, vaccines, and more that the country needs to prepare for future health emergencies. As a result, HHS was immediately able to offer support to states. To date, we have delivered nearly 4 million pieces of PPE and thousands of antivirals to protect workers. USDA also set up a program that reimburses farmers when they purchase PPE for their workers, and post-exposure prophylaxis with Tamiflu is also promptly offered to workers with any known exposure. We have also taken steps to build trust with impacted communities along the way – investing $5 million in campaign to educate and test farmworkers. In total, USDA and CDC have deployed over 100 federal workers into the field to support response and support workers.
As we protect workers today, we are also preparing for any possible scenario tomorrow. The CDC and NIH are tracking changes in the virus so we can see whether it’s becoming more adaptable to humans. We have already prepared nearly 5 million doses of vaccines so they’re ready if we need them. Further, by the end of the first quarter of 2025, we will have stockpiled 10 million doses of vaccine to inoculate humans against bird flu. And we’ve invested $176 million in Moderna to develop next-generation mRNA vaccines that can rapidly respond to any adaptations in the virus, with phase 3 trials beginning shortly. In addition to vaccines, we have 68 million courses of influenza antivirals on hand in the Strategic National Stockpile to treat those who may become infected with the virus. We have made 3,000 courses available to affected communities.
Preserving Animal Health: Research suggests the virus travels via surfaces related to normal business operations such as vehicles, milking equipment, and people’s clothing. That’s meant that biosecurity practices—like limiting visitors, disinfecting work apparel, and separating animals of different species— were essential to reduce the spread and keep cows healthy. In early May 2024, USDA launched assistance for producers with H5N1 affected premises to improve on-site biosecurity in order to reduce the spread of the virus. This includes financial support for protecting workers with PPE, funding for disposal of milk, reimbursement for veterinary costs, and payments for shipping laboratory Samples. As of January 2025, over 500 farms have utilized these programs to date. Later that month, USDA expanded support for producers to stop the spread through cattle, by issuing a rule to compensate eligible producers with positive herds who experience a loss of milk production. So far, over 300 producers have applied to the program with tens of millions of dollars in payments distributed. Additionally, USDA accelerated efforts to develop a first-of-its-kind bovine vaccine for the virus, and candidates have already entered field safety trials. USDA also announced in early January 2025 that work would begin to build a new stockpile of avian flu vaccines for poultry.
Ensuring the Safety of Our Food Supply: We have 100 years of data showing pasteurization works, but it was essential for our Administration to confirm that this was still the case with this new pathogen. USDA and the Food & Drug Administration (FDA) began testing retail dairy samples from across the country to ensure the safety of the commercial pasteurized milk supply and conducted laboratory experiments to reaffirm that pasteurization inactivates the virus. USDA similarly conducted research to confirm that cooking beef to proper temperature inactivates the virus, which it does. USDA also sampled muscle tissue from culled cattle at beef processing facilities as part of our robust ongoing surveillance programs. Today, we are confident that pasteurized milk, as well as properly cooked meat and eggs, are safe for consumers.
Mpox, Marburg, Measles and More: Managing Additional Public Health Threats
The Biden-Harris Administration has also mounted a robust response to other infectious disease threats that have emerged since 2021, including Marburg and Mpox. On Day 1 of the multiple Marburg and Mpox responses, medical countermeasures existed and were ready to be deployed at home and around the world as a result of U.S. preparedness efforts. In the case of Marburg, the United States had invested in experimental vaccines and therapeutics in order to be able to quickly deploy, test, and eventually seek regulatory approval for new countermeasures.
Mpox Domestic and Global Responses:
In early 2022, an outbreak of clade II mpox (then known as Monkeypox) rapidly spread globally and domestically. Shortly after the first U.S. case was identified in May 2022, the Administration deployed tens of thousands of doses of an FDA-approved vaccine and hundreds of courses of an investigational therapeutic from the Strategic National Stockpile to support domestic efforts to control spread and treat patients. We also rapidly scaled up testing capacity from 6,000 to 80,000 tests per week and, by October 2022, over one million doses of JYNNEOS had been administered to individuals at heightened risk of exposure to mpox, over 40,000 treatment courses had been distributed across the country, and domestic cases of clade II mpox had decreased by 90%. Today the mpox vaccine, which is effective against both clades of mpox, is also now commercially available with an ample supply at health departments and local pharmacies.
During the 2022 response, the Biden-Harris Administration also stood up a White House National Monkeypox Response Team and, in collaboration with a diverse group of community-based and civil society partners, promoted equitable access to vaccines, therapeutics and testing and made significant strides in reaching vulnerable populations where they were with trusted community messengers. Among the many successes of this group were the planning and execution of multiple “pop up” sites at events where at-risk individuals could learn more about mpox and, if they chose to do so, protect themselves by getting vaccinated against mpox.
When a new clade of mpox began spreading internationally in 2024, the Biden-Harris Administration was poised to act quickly. Within weeks of WHO’s declaration of a public health emergency of international concern, President Biden announced in September 2024 that the United States was prepared to commit at least $500 million and to donate up to one million doses of mpox vaccines to support African countries in preventing and responding to this outbreak. We are delivering on that commitment, with two-thirds of our global mpox funding pledge fulfilled already, and all one million of the pledged mpox vaccine doses available now for countries that are ready to receive them.
Most biological threats emerge outside the United States, which means that Americans will be safer when countries around the world are prepared to prevent, detect and respond to threats when they emerge. As part of the implementation of the National Biodefense Strategy, the United States Government continues to work with more than 50 countries around the world – including most mpox-affected countries and those at-risk of an mpox outbreak – to build stronger global health security capacities, ensuring countries are better prepared to stop outbreaks at their source while protecting U.S. national and homeland security.
Domestically, in 2024, the Administration has continued to focus on additional preparedness steps to increase awareness of mpox risks; provided updated recommendations to prevent, detect, and treat both clades; and expanded wastewater surveillance to provide an early warning of mpox activity and community spread. In addition, the United States continued to build on the critical testing landscape created during the 2022 outbreak and can not only detect both clades of mpox, but can also differentiate between clade I and clade II mpox.
Global Marburg Response:
In September 2024, Rwanda’s Ministry of Health announced an outbreak of Marburg Virus Disease (MVD), a rare, viral hemorrhagic fever similar to Ebola. Over the last four years, the Biden-Harris Administration has responded to 11 Ebola or Marburg outbreaks. Immediately after learning of the outbreak in Rwanda, in partnership with the Government of Rwanda, the United States committed millions of dollars to address urgent health gaps in Rwanda and surrounding countries, through provision of technical assistance with surveillance and contact tracing, infection prevention and control guidance, case management, risk communication and community engagement, safe and dignified burials, donation of laboratory test kits, and point of entry exit screening at Rwanda’s airport and neighboring border crossings. Within 8 days of learning of the outbreak, the United States Government worked with the Government of Rwanda, WHO, CEPI, and other critical partners to share experimental vaccine doses with Rwanda, and into the arms of healthcare workers at high risk of exposure, an unprecedented public health achievement. The United States also contributed tests, treatments and PPE, to support response efforts and protect health workers. This support has been made possible through the United States robust investments in science and research over the last 10 years. On December 20, the outbreak was declared over by the Government of Rwanda, with one of the lowest case fatality ratios (22%) of any Marburg outbreak in history.
Domestic Measles Response:
As the number of children protected from measles infections continues to decline due to declining vaccine coverage and misinformation, multiple jurisdictions have had to rapidly respond to measles outbreaks. The administration has deployed experts to support local responses and has distributed additional vaccine doses to support targeted vaccination campaigns, which are effective in ending outbreaks. The Administration has underscored that while the measles vaccine is highly effective and provides durable protection, 93-95% vaccine coverage is needed to maintain community protection. An unvaccinated individual exposed to the virus has a 90% chance of developing disease – therefore vaccination is critical to this response.
6) Building the Infrastructure for Future Biological Threats
The Biden-Harris Administration has also taken historic actions, building on policies from prior administrations, to protect Americans from biological threats that may emerge in the future.
Replenishing the Strategic National Stockpile:
The Strategic National Stockpile (SNS) was created in 1999 to “provide for the emergency health security of the United States …in the event of a bioterrorist attack or other public health emergency.” Historically, the SNS holds vaccines and therapeutics to protect the country from any number of chemical, biological, radiological, or nuclear events. Many of these medical countermeasures are not commercially available and the SNS is the only source for these critical supplies in the country–and in some instances the world. Under the Biden-Harris Administration, the SNS’s budget increased by 25% – allowing it to secure more of the vaccines, therapeutics and medical supplies needed to protect the country from public health emergencies and disasters.
Since the beginning of the COVID-19 pandemic, the Strategic National Stockpile distributed more than 27,000 tons of medicines, equipment, and supplies to support the country’s public health and health care needs. Early in the pandemic, the Strategic National Stockpile (SNS) deployed 90% of its overall inventory of personal protective equipment (PPE) – nearly 72 million items – as well as 100% of its Federal Medical Stations, which served as alternate care sites across the country. Much of this PPE was purchased ten years before with funds from the H1N1 outbreak—and had not been restocked.
Since President Biden took office, the SNS has dramatically increased its stockpiled quantities of PPE and ventilators—with supplies that were made in America where possible. The SNS now has 70 times the number of N95 respirators, 34 times the number of gloves, 50 times the number of isolation gowns, and 10 times the number of ventilators than it had before the pandemic. Restocking the SNS to these levels has allowed it to make PPE available to communities impacted by COVID1-9, H5N1 and other infectious disease outbreaks. In 2022, the SNS provided nearly 300 million N95 masks to retail pharmacies and community health centers for free —the largest deployment of personal protective equipment in U.S. history. Under President Biden, the SNS has also assigned staff to state public health departments and completed a series of tribal consultations and urban Indian confers to ensure all communities understand what tools it has available and how to access them in times of emergency or disaster.
Expanding Surveillance Capabilities:
At the start of the COVID-19 pandemic, because of years of underinvestment in modernizing data systems, some state, local, tribal, and territorial jurisdictions still relied on fax machines to transmit public health data and the U.S. struggles to collect, analyze and share essential data on the presence of SARS-CoV-2 in communities, the rate of transmission, and the impact on hospitals. Throughout our response, the U.S. government has expanded our surveillance capabilities to monitor disease and better inform the public. These steps include:
Increasing the number of healthcare facilities which provide automated, near real-time electronic case reporting to local, state, tribal, territorial and federal public health officials from less than 200 in 2020 to over 48,000 in 2024 and supporting public health authorities to adopt the Trusted Exchange Framework and Common Agreement (TEFCA) to further enable the exchange of public health data across the healthcare ecosystem to help rapidly identify emerging outbreaks.
Standing up a National Wastewater Surveillance System, which routinely reports early warning information from over 1,500 sites covering over 150 million people in the United States.
Scaling up genomic sequencing, which is important to detect new pathogens including variants. At the start of the COVID-19 pandemic, only 23 public health labs demonstrated advanced molecular detection surveillance capabilities. By the end of 2022, this number expanded to 68 public health laboratories, and CDC launched 5 Pathogen Centers of Excellence. The average turnaround time for public health laboratories to publish genome sequences has dramatically improved – decreasing from 96 to 40 days. Some laboratories able to sequence in less than two days.
Developing a COVID-19 Variant Playbook, which served to assess the disease severity and transmissibility of a new variant immediately, and to expedite the rapid laboratory evaluation of the effectiveness of vaccines, tests, and treatments against any variant.
As a result of close collaboration between the CDC, the Centers for Medicare and Medicaid Services and industry stakeholders, over 80% of all hospitals from across America are now sharing critical data on emergency department visits and hospital admissions.
Advancing Capabilities in RNA Vaccine Technologies:
With investments totaling over $400 million, the Administration has also been advancing capabilities in RNA vaccine technologies to guard against future pandemics. To further these efforts, multiple companies are currently partnering with HHS to develop RNA vaccines that may allow for a faster, more sustainable response capability against multiple threats, lower the requirement for needles, simplify storage requirements, or investigate new methods of administration.
Strengthening Domestic PPE Supply Chain:
Given gaps in domestic capacity for critical PPE, this Administration, in an effort to minimize foreign reliance, the President signed the “Make PPE in America Act” in 2021. The statute requires that the Departments of Homeland Security (DHS), Health and Human Services (HHS) and Veterans Affairs (VA) (collectively referred to as “covered agencies”) issue long-term contracts for PPE containing only materials and components that are grown, reprocessed, reused or produced in the U.S. This requirement is critical to strengthening our domestic manufacturing capabilities and promoting the production of essential PPE in the United States. The statute recognizes the power of leveraging the federal government’s buying power as a catalyst to increase market participants, support competition and the health of the domestic PPE industry.
A sustained federal commitment to procure PPE from domestic sources supports the health of the domestic PPE industry. Since the enactment of the Make PPE in America Act, covered agencies took actions to support a long-term domestic federal procurement strategy, model demand, and more closely align their acquisition strategies to send a government-wide demand signal to the PPE industry, while working collaboratively with the Defense Logistics Agency (DLA) and the Office of Management and Budget (OMB) to implement the Make PPE in America Act.
Stopping Outbreaks at their Source and Transforming our Biopreparedness Capabilities:
In 2022, President Biden signed National Security Memorandum 15 on Countering Biological Threats, Enhancing Pandemic Preparedness, and Achieving Global Health Security. This NSM launched a new National Biodefense Strategy and Implementation Plan, which envisions “a world free from catastrophic biological incidents” and directs a coordinated, whole-of-government effort to prepare for biothreats. It includes an ambitious five-to-ten-year vision for developing moonshot biodefense capabilities, including the ability to develop new vaccines within 100 days and repurpose therapeutics within 90 days.
The most effective way to limit the impact of biological threats is to stop them at their source. The United States is working with countries and partners around the world to ensure they have the capacity to identify and stop emerging threats before they grow into regional or global threats. To advance that goal, the administration published an updated Global Health Security Strategy, laying out our commitment to working with foreign partners in order to continue building our capacity to prevent, detect, and respond to biological threats wherever they emerge. The Administration took several concrete actions to support these transformative efforts. The U.S. Government reached the ambitious goal of directly supporting 50 countries in building their health security capacity, which is one of the most powerful prevention tools we have in this space. The United States has also leveraged financial resources and diplomatic channels to mobilize support for 50 additional countries to strengthen their health security capacities, for a total of more than 100 countries receiving support.
Limitations in the existing global systems to finance pandemic prevention, preparedness, and response left countries and financial institutions ill prepared to effectively contain COVID-19. On day one, President Biden called on his Administration to transform the existing financing institutions and to cultivate new financing sources for global health security that are more effective and sustainable, and that are less dependent on U.S. government assistance. The United States was instrumental in the creation of the Pandemic Fund in 2022, the only multilateral financing facility dedicated exclusively to pandemic preparedness financing for low- and middle- income countries. The Pandemic Fund made significant progress in its first two years, awarding grants totaling $885 million, which mobilized an additional $6 billion in investments, to support 75 countries and economies across six geographic regions. The United States is also working to evolve Multilateral Development Banks to be better equipped to respond to the increasing frequency, scope, and complexity of global challenges, including pandemics. The Biden-Harris Administration strongly supported the establishment of the International Monetary Fund Resilience and Sustainability Trust and its goal of supporting low-income and vulnerable middle-income countries to access long-term, affordable financing to address longer-term challenges, such as health emergencies. During the COVID-19 pandemic, many countries and institutions lacked the liquidity to procure the medical countermeasures (MCM) needed to mount effective and timely responses. The U.S. Development Finance Corporation helped develop and lead a G7 Surge Financing Initiative, through which G7 development finance institutions (DFIs), the European Investment Bank, the International Finance Corporation, and global and regional health stakeholders are developing and deploying innovative financing tools to accelerate access to MCMs in health emergencies.
While there will always be new or evolving biological threats, developing effective countermeasures for known threats is a critical piece of preparedness. For example, the U.S. government invested billions of dollars in mRNA technology in advance of the COVID-19 pandemic. These public investments translated into millions of lives saved in the United States and around the world, and were crucial to developing the mRNA vaccine technology that can be leveraged in a future pandemic, as well as potentially treating other diseases. For example, the Department of Defense and the National Laboratories are leveraging Artificial Intelligence for rapid development of medical countermeasures. The Administration is executing a whole-of-government implementation plan for strengthening capabilities in early warning, vaccines, therapeutics, diagnostics, clinical trials, and PPE.
Strengthening Research Capacity and Oversight:
The lives saved and hospitalizations avoided as a result of the COVID-19 therapeutics and vaccines were the result of decades of foundational research investments. Because viruses and bacteria are constantly changing and the frequency of naturally occurring biological outbreaks is increasing, the Biden-Harris Administration published the American Pandemic Preparedness Plan in the fall of 2021. In addition, the administration has continued to invest more than $7 billion annually in research by Federal, academic and industry researchers in new ways of protecting Americans from tuberculosis, HIV and other infectious diseases that remain leading causes of illness in our nation. These investments are crucial to ensure our nation is prepared for future threats and to deter those who are seeking asymmetric advantages over the US.
In 2024, the Administration also released an updated policy to enhance oversight of research. This update to standards that were originally released in 2012 will reduce the likelihood of accidental outbreaks and deliberate misuse of life science research, while ensuring that lifesaving research proceeds.
Supporting Global Biosafety and Biosecurity:
Expanding biosurveillance capacity and the rapid evolution of technology are critical for health security, but can also elevate the risk of accidental and deliberate incidents. The Biden-Harris Administration has taken significant steps to minimize the chances of laboratory accidents; reduce the likelihood of deliberate use or accidental misuse; ensure effective biosafety and biosecurity practices and oversight; and promote responsible research and innovation. The Biden-Harris Administration knows that in order to protect the domestic population, investing in global biosafety and biosecurity practices and oversight is essential. These efforts – which minimize the chances of laboratory accidents, reduce the likelihood of deliberate use or accidental misuse, and more – have been critical as we expand biosurveillance capacity. For example, the United States secured inclusion of biosafety and biosecurity as a critical component of the Pandemic Fund grants to support laboratory systems. One of the projects, the Caribbean Public Health Agency Train-the-Trainer Workshop on the Safe Transportation of Infectious Substances, resulted in certified trainers well-positioned to serve as national trainers and advisors in biosafety and safe transport protocols, ensuring safer practices across the region. The U.S. global health security bilateral partnerships also build capacity in biosafety and biosecurity: the final global health security report of the Administration showed that global health security partner countries with at least two years of U.S. Government support demonstrated a net improvement in biosafety and biosecurity capacity from 2018 to 2023. The Administration also supported the 2024 World Health Assembly resolution on Strengthening Laboratory Biological Risk Management, which calls for improvements to biosafety and biosecurity practices globally.
Standing up the Office of Pandemic Preparedness and Response Policy:
Recognizing the growing threat of pandemics, the Administration stood up the Office of Pandemic Preparedness and Response Policy (OPPR) in August of 2023. This is a permanent office in the Executive Office of the President charged with leading and coordinating actions related to preparedness for, and response to, known and unknown biological threats or pathogens that could lead to a pandemic and/or to national security. OPPR assumed the duties of the COVID-19 Response Team and Mpox team at the White House and has continued to coordinate and develop policies and priorities related to pandemic preparedness and response.
Because every successful response to a biological event has required a synchronized, integrated team, OPPR has worked with federal, state and local health partners, and has engaged with a broad array of private sector, academic and other stakeholders to ensure lessons from the response to COVID-19 and other recent outbreaks inform future plans and response efforts. Recent accomplishments include:
Partnering with multiple industry stakeholders to resolve supply chain issues affecting the availability of a new immunization, reducing infant hospitalizations by more than 80%.
Partnering with leaders from the European Union, the Republic of Korea, Japan, India and multiple industries to develop a process to quickly recognize and address supply chain issues affecting availability of medications in the US and partner countries.
Partnering with leaders from the Long-Term Care industry to improve vaccination rates for residents of long-term care facilities.
Partnering with leaders from across the domestic healthcare enterprise to identify and mitigate constraints to our national preparedness for future biological events and to create a collaborative committed to continuing to mitigate future challenges.
Partnering with community support organizations to identify best practices from the COVID-19 pandemic and to develop strategies to sustain outreach to historically medically underserved communities to ensure all Americans can access their healthcare options.
Conclusion
As of result of the Biden-Harris Administration’s efforts, the COVID-19 pandemic no longer disrupts our daily lives, our children are back in schools, our economy is stronger than ever and families have been able to resume their pre-pandemic routines. As new viruses and other biological threats have continued to emerge, we have remained vigilant in monitoring and responding to each threat. From investments into research for new tests and treatments for diseases, to launching the largest vaccination program in our country’s history, to expanding the nation’s surveillance capabilities, to replenishing the Strategic National Stockpile, the Biden-Harris Administration has transformed our nation’s pandemic preparedness and response.
In order to ensure that future Administrations are prepared for any threat that emerges, the Biden-Harris Administration will also leave behind a three-step internal playbook with nearly 300 pages of guidance on how to rapidly and effectively respond to biological threats from all sources – naturally occurring, accidental and deliberate.
Step 1: Within 24 hours of notification of a serious biological threat, NSC convenes Departments and Agencies for a biological incident notification and assessment (BINA).
Step 2: If the threat poses a significant risk to the United States, within 24-48 hours Departments and Agencies establish an Incident Response Coordination Structure (IRCS), with agency leadership and support roles pre-determined in a playbook.
Step 3: Departments and Agencies operationalize a rapid and effective response, with coordination by the IRCS and leadership from the White House. The playbook includes detailed operational annexes to address many scenarios that commonly arise during biological incident responses.
Under the Biden-Harris Administration, we have exercised this three-step playbook numerous times, including in recent weeks. All Departments and Agencies have received final versions of the Playbook, and the Biden-Harris Administration will give copies to the incoming Administration to ensure they are prepared to act on day one of a crisis to protect the American people.
###
State Public Health Data Reporting Policies and Practices Vary Widely
Most health care providers already use electronic health records (EHR) to manage their patients’ medical information. Public health agencies use the data to monitor and detect threats, and design strategies to reduce disease spread and impact. The report is informed by the direct examination of each jurisdiction’s public health statutes and regulations (conducted from May to August 2021) and by interviews with 266 public health officials in the District of Columbia. Researchers designed the interviews to supplement the policy analysis with more on-the-ground feedback about how these policies are implemented. Together, the findings about policy and practice provide a comprehensive view of what is driving data modernization. For more information, visit the National Institute of Health and Human Services (NIH) and the National Association of Public Health Agencies (NAPHA) for more information on how to use the report to help you understand public health and health care policies and practices. For the full report, go to: http://www.nah.org/research/reports/public-health-and-health/reports.html.
When public health agencies lack access to clinical data, illnesses spread undetected, the health system becomes overburdened, and health care costs, illnesses, and deaths rise. The water crisis in Flint, Michigan, and the COVID-19 pandemic demonstrate shortcomings in the collection of public health data and their ramifications.1
By collecting and analyzing better data, public health agencies can more effectively identify and prevent the spread of emerging threats; anticipate surges of illnesses and deploy resources where they are needed most; identify communities that are disproportionately threatened by disease and lack access to care; and design more effective and equitable interventions. Agencies can also share more information—with health care providers to improve clinical practice, with scientists to aid research, with elected officials to inform policy, and with members of the general public to guide their daily behavior.
Most health care providers already use electronic health records (EHR) to manage their patients’ medical information. By employing those products to automatically report data about infectious diseases and other health threats to public health agencies, health care providers can significantly reduce or eliminate burdensome effort on their part to report and improve the timeliness, accuracy, and completeness of the data they share. Unfortunately, a sizable proportion of case data is still reported via manual processes such as fax and phone, which creates administrative work, introduces human errors, and slows the analysis and use of information. And when diseases spread hour by hour and day by day, speed matters.
Policies and practices designed to drive automated electronic reporting vary among providers and states. To modernize public health data, federal and state policymakers, public health officials, health care providers, and health IT developers need a baseline understanding of how jurisdictions are regulating and promoting automated electronic reporting
This report from The Pew Charitable Trusts provides a first-of-its-kind analysis of these policies and practices across all 50 states and the District of Columbia (referred to as jurisdictions throughout the report) that govern how clinical data flows to state public health agencies via four methods: case reports, lab reports, syndromic surveillance, and immunization information systems.
Case and lab reports provide state and local health departments with information about individual patients with conditions of public health importance, such as communicable diseases, environmental illnesses, or other conditions of public health importance (for example, some types or instances of cancer or lead poisoning). 2 Public health agencies use the data to monitor and detect threats and design strategies to reduce disease spread and impact.
Public health agencies use the data to monitor and detect threats and design strategies to reduce disease spread and impact. Syndromic surveillance is an early-warning system that uses de-identified reports (with identities redacted) of symptoms and syndromes captured primarily from emergency departments. The data enables agencies to detect and identify emerging threats quickly and perform real-time monitoring of known health risks.
Immunization information systems collect data on individual vaccinations—especially for childhood vaccines—to track immunization rates, identify communities that may lack access to vaccines, and investigate cases and outbreaks of vaccine-preventable diseases. Patients and providers also use the systems to keep track of their immunization records.
The report is informed by the direct examination of each jurisdiction’s public health statutes and regulations (conducted from May to August 2021) and by interviews with 266 public health officials in the District of Columbia and every state except Kansas, Maine, and Wyoming (conducted from October 2022 to April 2023). Researchers designed the interviews to supplement the policy analysis with more on-the-ground feedback about how these policies are implemented. Together, the findings about policy and practice provide a comprehensive view of what is driving data modernization. Although these interviews covered topics related to jurisdictions’ exchange of data with health care providers and other government institutions, researchers interviewed only state officials; thus, this report does not reflect the perspectives of health care providers or federal, local, tribal, or territorial institutions, except for the District of Columbia.
Among the key findings:
Each state has unique needs and capacities. There is no single approach to modernizing public health data, but jurisdictions can still learn from each other and adopt common approaches and solutions.
Federal and state agencies that govern public health data can do more to improve reporting by enabling and encouraging health care providers to meet requirements, rather than through enforcement. For example, although only 13 jurisdictions require syndromic surveillance reporting, participation is widespread nationally, in part because of Medicare incentives.
State public health officials commonly cited staffing shortages, outdated IT infrastructure, and inadequate funding as the reasons for not investing in modern IT systems and expert staff to collect, analyze, and use electronic data.
State public health officials said some providers—including smaller and rural providers—do not have the resources to invest in automated electronic reporting systems, and officials are concerned that requiring them to do so may discourage reporting altogether. For smaller providers, some jurisdictions have created alternative methods to enable them to report more data electronically without having to adopt expensive technologies.
Positive signs demonstrate that more case reports can be automated and electronic, as opposed to manually transmitted via fax and phone. This will help improve the timeliness and accuracy of the data that public health agencies need to detect and prevent diseases more effectively.
Policymakers and public health practitioners can take some steps to modernize public health data reporting.
States should measure their baseline performance on data reporting—such as automated electronic reporting as a proportion of all reports, data timeliness, and completeness—to prioritize areas that need the most improvement, inform policies and strategies, and track progress. Officials could also benefit from engaging their counterparts in peer states to better understand how policy, staffing, funding, and other practices affect data quality. States can also share their experience with federal agencies to inform national data-modernization efforts.
Federal and state policymakers should explore ways to improve data from under-resourced health care providers that cannot afford automated electronic systems, such as incentivizing the use of web portals or batch uploads for providers that report at low volumes. These systems are not fully automated, but they can still reduce the burden on providers and public health agencies and improve data quality.
As the federal government works to standardize public health data exchange, it should engage health care providers and public health agencies to ensure that the incentives, standards, and processes they design are practical and achievable for a broad array of data reporters and users. In addition, state and local governments should provide their public health agencies with the resources needed to participate in federal data-modernization efforts.
Glossary
These terms are defined in the context of public health data reporting. Some may have different connotations in other fields of health and technology.
Automated electronic reporting. The use of health information technology (e.g., electronic health record systems) by health care providers (such as doctors, pharmacists, vaccine administrators) to send clinical data (e.g., symptoms, diagnoses, treatments, outcomes, demographic details) to state, tribal, local, territorial, and/or federal public health agencies.
Batch upload. Transmitting data files that contain large sets of clinical data, typically for more than one patient and optimally in standardized formats that allow for rapid processing and analysis.
BioSense Platform. Defined by the Centers for Disease Control and Prevention (CDC) as “a cloud-based early detection and monitoring system that helps the public health community protect Americans from injuries, emerging diseases, and environmental disasters.” See also National Syndromic Surveillance Program (NSSP).3
Case report. A set of individualized patient data generated by a clinical care organization and submitted to a public health agency as required by law or regulation. It often includes symptoms, lab results, diagnoses, treatments, outcomes, demographic data, and other details extracted from an electronic health record.
Data Modernization Initiative. The CDC’s “multi-year, multi-billion-dollar effort to modernize data across the federal and state public health landscape.”4
Electronic case reporting (eCR). Per the CDC, “the automated, real-time exchange of case report information between electronic health records and public health agencies.”5 The general, unabbreviated term may refer to any electronic technology used to transmit clinical case data but not necessarily via automated systems. All eCR technologies fall into the category of electronic case reporting, but not all electronic case reporting qualifies as eCR.
Electronic health record (EHR). Per the Office of the National Coordinator for Health IT (ONC), “a digital (computerized) version of patients’ paper charts. … EHRs are real-time, patient-centered records. They make information available instantly, ‘whenever and wherever it is needed.’ And they bring together in one place everything about a patient’s health.”6
Electronic initial case report (eICR). A standards-based electronic file that is generated by an EHR and transmitted to a public health agency to support eCR.
Electronic lab reporting (ELR). According to the CDC, ELR is “the transmission of digital laboratory reports from laboratories to health care and public health partners.”7
Electronic reporting. The use of any electronic tool to report data to public health. All eCR and ELR technologies fall into the broader category of electronic reporting, but states may define electronic reporting in policy in broad ways that include manual technologies such as fax or email.
Flat file. A single table of data in a simple, text-based format.
Health care provider. A trained and licensed clinical care professional (for example, primary care physician or nurse practitioner, specialist, school nurse, pharmacist, vaccine administrator) and/or facility offering health care (such as a hospital, emergency department, pharmacy, school). Throughout the report, context indicates when the term refers to individuals, hospitals, and/or health systems.
Health equity. The guiding principle that health is a fundamental human right and that all people—regardless of their identity, income, or location—should have access to the resources they need to be as healthy as possible, such as effective medical care, quality education, safe and affordable housing, nutritious food, and clean air and water.
Health information exchange (HIE). An organization or technological platform that facilitates the electronic sharing of health data, in compliance with all applicable patient privacy laws. It may also refer to the act of exchanging data via those organizations or platforms.
Health Information Portability and Accountability Act (HIPAA). The 1996 federal law that establishes the rights of patients to protect the privacy and confidentiality of personally identifiable information in their medical and health insurance records.8 It allows patients to access their own records and health care providers to share certain information with authorized recipients, including public health agencies when states designate the information as reportable.
Health Level Seven International (HL7). A not-for-profit “standards developing organization dedicated to providing a comprehensive framework and related standards for the exchange, integration, sharing and retrieval of electronic health information that supports clinical practice and the management, delivery and evaluation of health services.”9
Immunization Information Systems (IIS). Per the American Immunization Registry Association, “previously known as immunization registries, [IIS] are confidential, population-based, computerized databases that record all immunization doses administered by participating providers to persons residing within a given geopolitical area.”10
Interoperability. The ability of two or more electronic systems to exchange health information and use the information once it is received.
Lab report. A set of data that a lab generates and submits to a public health agency that may include lab test orders from health care providers, results, and other patient information.
Manual reporting. The use of fax, phone, mail, and other technologies to report case, lab, or immunization data to public health agencies. As opposed to automated electronic reporting, these processes require health care providers and public health officials to enter, transmit, receive, and process the data, which causes delays and may introduce errors.
National Syndromic Surveillance Program (NSSP). The CDC’s effort to collect electronic data from emergency departments across the United States. It collects and manages the data via the BioSense Platform.11
Notifiable diseases. Diseases and other health conditions that state and local health agencies report to the CDC.
Policy. Unless otherwise noted in this report, it refers to the CDC’s definition: “a law, regulation, procedure, administrative action, incentive, or voluntary practice of governments and other institutions.”12
Promoting Interoperability Program. A Medicare program that uses reimbursement incentives to promote public health data reporting via electronic health records that are certified by the ONC.
Reportable diseases. Diseases and other health conditions that health care providers must report to state, tribal, local, or territorial public health agencies, usually communicable diseases and environmental hazards that can pose a significant health threat. State, tribal, local, or territorial policies designate which diseases must be reported.
Reporters/data reporters. Health care providers who submit clinical data to public health agencies
Surveillance/disease surveillance. The public health practice of using data to detect and monitor emerging and existing threats, especially communicable diseases.
Syndromic surveillance. An early-warning system that uses de-identified reports of symptoms and syndromes captured primarily from emergency departments. The data enables agencies to detect and identify emerging threats quickly and perform real-time monitoring of known health threats.
Key findings and themes
The following table summarizes data-reporting policies of the 50 states and District of Columbia.
Table 1 Case Lab Syndromic Immunization Where is reporting required in statute or regulation? 51 jurisdictions 51 jurisdictions 13 jurisdictions* 34 jurisdictions for all ages; 11 jurisdictions for only childhood immunizations† Where is automated electronic reporting required in statute or regulation? 0 3 4 0 Where is electronic reporting required in statute or regulation? 3 7 4 5 What methods are specified as optional in statute or regulation? Phone, 46
Electronic, 34
Fax, 25
Mail, 19
Email, 3
Web portals, 3
Automated
electronic, 2 Phone, 38
Electronic, 27
Fax, 20
Mail, 16
Automated
electronic, 7
Web portals, 6
Email, 2 Automated
electronic, 2
Electronic, 1
File transfer protocol, 1
Batch messages, 1 Electronic, 14
Automated
electronic, 7
Web portals, 7
Fax, 1
Mail, 1
Phone, 1 Who is required to report data? Health care providers, 51
Hospitals, 45
Schools and school officials, 37
Child or day care centers, 30
Labs, 21
Correctional facilities, 14
Pharmacists, 13
Veterinarians, 10 Labs (including in hospitals), 51
Health care providers, 2 Emergency departments, 10
Urgent care centers, 3
Health care providers, 2
Inpatient facilities, 2 Pharmacists, 37
Health care providers, 32
Hospitals, 15
Schools and school officials, 11
Child or day care centers, 8
Insurers or health plans, 7 * This excludes states that collect syndromic surveillance data under authorities established through broader statutes or regulation. † States that require reporting for children’s immunizations alone vary in how they define ages of children.
In addition to analyzing state policy on paper, Pew interviewed state public health officials to understand how those policies were implemented in practice. The following themes emerged from those interviews.
State and federal public health agencies can improve public health data reporting by learning from each other. Each jurisdiction has unique populations and diverse health priorities. Resources vary, too, which affects the capacity of health departments and health care providers to buy and implement modern data-management systems. Reflecting this diversity, states take a wide variety of approaches to enable, encourage, and require health care providers to share timelier, standardized, and accurate clinical data with public health agencies—and they have a diverse set of outcomes to show for it. There is no one-size-fits-all solution, but that does not mean 50-plus different solutions are necessary. Common approaches and solutions are useful to modernize data even within this variability.
States and the federal government do more by enabling and encouraging data reporters to meet requirements than by enforcing them. To compel reporting, nearly every state has enforcement mechanisms that range from fines to imprisonment. However, none of the interviewees could recall an instance in which any sanction was imposed on a noncompliant reporter. Instead, they said that informing providers about data quality, timeliness, and completeness—and sometimes escalating communications to more senior officials—often improved the issues without stronger action. Interviewees also reported that incentives, such as Medicare’s Promoting Interoperability Program, effectively improved reporting.
Automated electronic reporting has been shown to generate timelier, more complete, and more accurate data than manual reporting.13 However, the functionality and performance of the EHR systems and the implementation of technological data-sharing standards also significantly affect data quality. States do not necessarily collect better data if they require automated electronic reporting without also investing substantial time and effort into working with data reporters to evaluate and improve data quality.
Case reporting made strides during the COVID-19 pandemic but still needs urgent improvement. Although every state requires health care providers to notify public health agencies about infectious diseases, environmental illnesses, and other threats via case reports, officials in many jurisdictions said that cases were under reported and most likely to be reported for rare diseases or immediately reportable conditions (such as measles or meningococcal disease). As a result, officials said they tend to rely on lab reports, rather than case reports, as their primary source of information about emerging diseases. However, health departments need both case and lab data to have the most comprehensive and timeliest possible view of their communities’ health.
Further, when this research was conducted, health care providers and public health agencies widely used electronic case reporting (eCR) systems to report and collect COVID-19 data, but they continue to use fax, mail, and other reporting methods for other diseases. These older technologies require providers and practitioners to manually enter, transmit, and synthesize data, which slows analysis and introduces human error. A few jurisdictions are using eCRs for some additional conditions, but none is using it for all reportable conditions. Officials in about half of the jurisdictions Pew interviewed said that, although their state health departments receive some eCR, they do not incorporate the data into their surveillance systems. And even though most state health agencies and providers use technological standards such as Health Level 7 (HL7) to help automate data sharing, several officials said data quality still suffers and additional national standards are still needed.
By comparison, interviewees from about two-thirds of jurisdictions estimated that at least 90% of lab reports were transmitted via electronic lab reporting (ELR). Only two jurisdictions’ officials estimated the figure was less than 70%. Officials from a few jurisdictions did not provide estimates. Almost all interviewees estimated that they received most vaccination reports from health care providers—ranging from 50% to 95%—via automated electronic means. By design, virtually all syndromic surveillance data is reported by health care facilities via automated electronic systems.14
The rapid adoption of eCR during the pandemic for reporting COVID-19 and the increasing use of ELR over the past 20 years or so indicate that case reporting can shift from manual to automated electronic systems given enough time, funding, staff, training, and political will.
To ensure that reporting requirements do not exacerbate health inequities, public health officials want to accommodate providers with fewer resources. In interviews, officials in many jurisdictions said it is cost prohibitive for small and often rural providers to use their IT systems for automated electronic public health data reporting. They also said staffing shortages made it difficult for providers to adopt them. And officials in several jurisdictions said that some providers lacked adequate internet access to report data electronically. As a result, interviewees stressed the need for flexible reporting policies that can still improve data without setting unachievable requirements.
For example, during the pandemic, Oklahoma shifted away from receiving faxes of lab reports by establishing a laboratory reporting system that could still accommodate health care providers without ELR capabilities to submit lab results for COVID-19 via spreadsheet-type .CSV files or manual entry.15 The laboratory system was designed to check for missing data fields for all reporting methods. The state’s health department reported that this flexibility has significantly improved data completeness and it is considering expanding this approach for other conditions.
Most jurisdictions cited lack of fundamental resources to improve reporting. When asked to identify the biggest barriers to improving public health data exchange, most respondents said limited capacity, citing staffing shortages, outdated IT infrastructure, and inadequate funding. Without sustained and predictable funding, public health officials said they are reluctant to invest in the IT systems and expert staff that they need to collect, analyze, and use electronic data.
Because of the qualitative nature of the interview data presented throughout this report, specific terms are often used to refer to numerical ranges of jurisdictions. A “few” jurisdictions means three or four; “several” means five to 10; “many” means 11 to 30; “most” means 31 to 37; and “almost all” means 38 or more.
Background
Why does data matter to public health?
Just as doctors need data to diagnose and treat their patients, public health agencies rely on data to measure and improve the well-being of their communities.
Data enables public health agencies to provide essential services such as:16
Detecting and investigating infectious and environmental health threats.
Monitoring the health of the population.
Identifying health inequities, which the World Health Organization defines as “differences in health status or in the distribution of health resources between different population groups, arising from the social conditions in which people are born, grow, live, work, and age.” 17
Designing and implementing evidence-based policies and practices to prevent and manage illness, improve health, and reduce inequities.
Allocating funds and workforce more efficiently and effectively.
Evaluating the impact of their interventions and adjusting as needed.
In turn, individuals and organizations rely on information from public health agencies, including health care providers who use it to guide clinical practice, scientists to conduct research, elected officials to develop policy, and the general public to guide their daily behavior.
Where do public health agencies get their data?
Public health agencies receive data from a variety of sources within and outside the health sector, such as emergency departments, pharmacies, and schools. Doctors’ offices, hospitals, health systems, and labs generate troves of data from millions of daily patient interactions.18 This report examines the state-level policies and practices that govern how clinical data flows to state public health agencies via four streams: case reports, lab reports, syndromic surveillance, and immunization information systems.
Case reports provide state and local health departments with information about individual patients with communicable diseases, environmental illnesses, or other conditions of public health importance (e.g., cancer, lead poisoning).19 Generated and shared by health care providers, these reports help agencies detect threats, conduct surveillance, and design strategies to reduce their spread and impact. Public health agencies can use demographic information in case reports (e.g., age, sex, race, ethnicity, ZIP code) to identify communities and populations at heightened risk of different diseases and most urgently in need of public health intervention.
Lab reports are based on orders from health care providers on tests that a lab performs. Labs send results to state and local health departments when patients’ results are positive for conditions of public health importance. In some cases, negative test results are also reported for certain conditions, such as hepatitis C. Positive lab reports help public health agencies confirm the presence of a reportable disease, initiate investigations, and conduct surveillance of reportable diseases if agencies receive them before case reports. Public health agencies can identify emerging threats more quickly by analyzing lab test orders, which reflect preliminary diagnoses and can be reported days before the results are in.
Syndromic surveillance is often anonymous, derived from de-identified data captured during visits, admissions, discharges, and transfers in health care facilities, primarily emergency departments. This data includes patients’ chief complaints, such as shortness of breath or vomiting, and diagnosis codes. The system is designed for speed; it can enable officials to perform real-time monitoring of known health threats and detect and identify emerging threats earlier than they can with confirmed case and lab reports.
Immunization information systems collect data on individual vaccinations, especially for childhood vaccines. These systems are managed by states and local jurisdictions and help providers and health agencies track immunization rates, identify communities that may lack access to vaccines, and investigate cases and outbreaks of vaccine-preventable diseases. Patients and providers also use the systems to keep track of their immunization records.
Automation: A Key Factor in Electronic Data Reporting Electronic technologies can improve the flow of timely, standardized, and complete data, but not always. It depends on how states define “electronic,” if they do so at all. Some count fax and email as electronic technologies, even when these kinds of reporting require significant manual input. Automation is the key factor that makes electronic reporting optimally effective. Often piggybacking on certified electronic health record systems, automated electronic data reporting requires limited to no manual input or reformatting from the health care entity sending the data. This can help ensure that the data is timely, standardized, and complete.
Some electronic technologies are partially automated. Web-based portals and digital spreadsheets (e.g., .CSV files), for example, may require providers to enter data manually but then allow public health agencies to receive it automatically in standardized formats for rapid processing and analysis.
Some electronic reporting processes feature no automation at all. This can include secure fax, email, or file transfers that require health care providers to manually input and transmit data to public health agencies, which must then manually reformat and reenter the data in their database. Manual input can introduce human error and delay analysis. The researchers defined automated electronic reporting for the report as an electronic data transmission from a reporter, such as an EHR or laboratory information management system, that integrates into a public health data system with minimal manual intervention. Many jurisdictions specify that automated electronic methods may be used to share data with public health agencies, but most still allow manual transmission. Some jurisdictions require automated electronic reporting for lab, syndromic surveillance, and/or immunization data; none mandates it for case reports. The findings in this report detail these differences.
How can public health agencies get the most value out of data?
To effectively detect, monitor, and prevent disease, public health agencies need data that is timely, standardized, and complete.20
Timely. Infectious and environmental threats can spread quickly. Public health agencies need data in real time (or as close to it as possible) to effectively trace the spread of diseases, limit the population’s exposure to them, and anticipate surges in illnesses and deploy resources (e.g., ventilators, masks, tests, vaccines, medication) where they are needed most.
Infectious and environmental threats can spread quickly. Public health agencies need data in real time (or as close to it as possible) to effectively trace the spread of diseases, limit the population’s exposure to them, and anticipate surges in illnesses and deploy resources (e.g., ventilators, masks, tests, vaccines, medication) where they are needed most. Standardized. To receive and analyze data quickly, public health agencies need it to be formatted consistently. This requires both sender and receiver to use interoperable technology (meaning different systems can exchange information seamlessly) and uniform conventions for labeling and recording data. When public health agencies receive information from multiple sources via different methods in different formats, it can take significant time, staff, and money to prepare the data for analysis and use.
To receive and analyze data quickly, public health agencies need it to be formatted consistently. This requires both sender and receiver to use interoperable technology (meaning different systems can exchange information seamlessly) and uniform conventions for labeling and recording data. When public health agencies receive information from multiple sources via different methods in different formats, it can take significant time, staff, and money to prepare the data for analysis and use. Complete. Public health agencies can detect and prevent diseases more effectively if they have comprehensive and granular data (e.g., sex, race and ethnicity, vaccination status, ZIP code) that enables them to identify communities disproportionately threatened by a disease or most in need of care. More narrowly and immediately, public health agencies also need accurate contact information to conduct case investigations, confirm illnesses, and identify risk factors; perform contact tracing; and connect people to treatment or prevention measures
Automated electronic reporting helps ensure that data meets these criteria. For example, a North Carolina study found that, compared with manually submitted reports, ELR produced more accurate information and allowed the recipients to process the data more efficiently.21 A study in Florida found that ELR nearly halved the time from symptom onset to health department reporting for salmonellosis and shigellosis, two infections once commonly reported by postal mail.22 And researchers found that New York City’s immunization data was significantly more timely and complete when it was exchanged electronically using a common technical standard (HL7) compared with manual transmission.23
How is health data regulated?
State oversight
States set most policies related to the reporting of public health data. A mix of statutes, regulations, and agency policies dictates:
Who must report data (e.g., physicians, hospitals, doctors’ offices, urgent care providers, labs, veterinarians, schools).
For which diseases or conditions reporters must share data (e.g., communicable diseases, environmental illnesses).
Time frame to report (e.g., immediately, 24 hours, seven days).
What types of data must be reported.
To whom reporters must report data.
The systems, methods, standards, and formats that must be used to report.
Patient privacy and confidentiality protections.
Penalties for failure to comply with reporting requirements.
That said, states do not always specify each of these elements. And further, some local governments layer additional reporting requirements over state policies.
Federal oversight
The federal government has significant influence but limited regulatory authority over the management of health data. While health care providers are required to report certain diseases to state public health agencies, notification from state to federal public health agencies is voluntary. Three agencies within the U.S. Department of Health and Human Services play a direct role in national efforts to modernize public health data reporting.
The Office of the Assistant Secretary for Technology Policy (ASTP), formerly known as the ONC, influences the development of health IT tools and standards.
As a condition of certification, it requires EHR products to allow their users to electronically share standardized health data with public health agencies.
It leads the development of the U.S. Core Data for Interoperability, which standardizes how data elements (e.g., name, address, diagnosis, treatment) are formatted, cataloged, and exchanged.
The office also oversees the Trusted Exchange Framework and Common Agreement, which provides a common set of legal agreements, infrastructure models, and governance structures that health care providers, health information exchanges, public health agencies, and other entities can use to share data.24
The Centers for Medicare & Medicaid Services (CMS) sets Medicare and Medicaid reimbursement policies that require participating health care facilities and providers (e.g., hospitals, physicians) to send clinical data to public health agencies.
Through its Promoting Interoperability Program and Merit-Based Incentive Payment System, Medicare provides participating hospitals and physicians with financial incentives to report case, lab, syndromic, and immunization data to state and local public health agencies.
During the pandemic, CMS required hospitals participating in Medicare and Medicaid to report COVID-19 case and hospitalization data (e.g., admissions, occupied and available beds) to the CDC, either directly or through their state’s health department.25
The CDC has broad responsibilities to protect the public’s health, including by collecting disease surveillance data from a wide variety of sources and analyzing it to inform public health decision-making. For example:
The agency maintains national disease reporting systems populated by data from local and state health departments and provides those agencies with funding and technical assistance to improve their collection, analysis, and use of the information.
It provides funding and grants to state and local health departments for data modernization as well as disease surveillance, prevention, and control activities for a wide range of conditions; it may require data reporting as a condition of funding. 26
The CDC has developed a Public Health Data Strategy and is facilitating data sharing between health care providers and public health agencies via the Data Modernization Initiative, a multiyear effort started in 2019 “to get better, faster, actionable insights for decision-making at all levels of public health.”27 It also develops and promotes data-sharing standards, such as HL7, which enables health care providers, health information exchanges, public health agencies, payers, and other organizations to securely share clinical data with each other even if they are using different electronic systems to manage and store the information.
Federal laws also set a floor for health privacy, chiefly via the Health Insurance Portability and Accountability Act of 1996 (HIPAA), which prohibits the disclosure of patients’ health information without their knowledge or consent. Balancing individual and societal needs, Congress designed the law with exemptions that allow health care providers, hospitals, and labs to share some patient information with public health agencies to prevent the spread of communicable diseases. Similarly, the Family Educational Rights and Privacy Act of 1974 protects the privacy of students’ education records, though it allows schools to share some data with health departments in cases of health and safety emergencies.28
Findings by data source
The next three sections present the findings of the legal analysis and interviews by data source: case and lab reporting, syndromic surveillance, and immunization reporting. Each section opens with some brief background information about the nature and value of the data source and presents key takeaways and action items. The complete findings follow, including jurisdictions’ general data-reporting policies and practices, its required reporters, required and acceptable reporting methods, how data flows from health care to public health, and how jurisdictions assess and improve data quality.
Case and lab reporting
When physicians first systematically reported diseases in the United States in 1874, they mailed postcards.29 Since then, doctors, hospitals, labs, and other health care providers used telegrams, phone, and fax to report data.
Today, for labs, automated digital systems are mature and used widely, providing lessons and inspiration for the future of case reporting, which still relies heavily on fax and phone.
Compliant with HIPAA privacy protections, case reports from health care providers can include a patient’s name, contact information, race and ethnicity, symptoms, diagnoses, treatments, and outcomes.30 Lab reports come from labs and are usually generated when people test positive for a reportable illness; they typically include less information than a case report. As happened during the COVID-19 pandemic, labs are sometimes required to report negative results.31
Case and lab reports are inherently delayed as it takes time for people to be exposed to an infectious or environmental threat, develop symptoms, see a provider, get diagnosed and tested, and then have their case reported. Manual reporting causes further delays as the paper-based reports often lack key information and require people to transfer the data into public health databases by hand, which also introduces human error.32
One promising solution is eCR, which automates the transmission of reportable data from EHRs to public health agencies. (See “Automation: A Key Factor in Electronic Data Reporting” on p. 10 for examples of what is automated and what is simply electronic.) Although most health care providers and organizations already use EHR systems to digitize their patients’ medical charts, eCR adoption is not as widespread, and barriers are slowing its adoption.33
Like eCR, ELR automates the reporting process, allowing labs to consistently and automatically transmit detailed, standardized data to public health agencies. Compared with eCR, however, ELR is more widely used. Case reporting made strides during the COVID-19 pandemic, and while eCR efforts have only recently gained momentum, the CDC has been supporting ELR in state and local health departments since 2010.34 All state health agencies have reported to the CDC that they receive at least some laboratory data via ELR.35
Key takeaways
Every jurisdiction requires case and lab reporting, but none requires eCR, three require generic (that is, not necessarily automated) electronic case reporting, only three jurisdictions require ELR, and seven require generic electronic reporting for lab results. Automated electronic reporting is an optional method specified in statutes or regulations in two jurisdictions for case and in seven jurisdictions for lab. Almost every jurisdiction specifies in statute or regulation when data must be reported (e.g., within 24 hours of seeing a patient) and what data elements must be included (e.g., date of birth, sex, age, race/ethnicity).
While the pandemic effectively forced health care providers and public health agencies to adopt eCR for reporting and collecting COVID-19 data, these automated electronic systems are still not widely used for other reportable diseases. 36 (See “Lessons Learned From the COVID-19 Pandemic” on p. 42 for more information about how states modernized eCR during the pandemic and are applying the lessons for other diseases.) Worse still, public health officials in many jurisdictions said that cases are underreported overall, with providers more likely to report cases of rare diseases or immediately reportable conditions. As a result, public health agencies rely more on lab reports to create their internal cases, rather than requiring both case and lab reports. However, public health agencies that rely on labs for data may be missing patients who have a reportable illness but did not take a lab test. In addition, lab reports often lack key details (e.g., race and ethnicity, onset date, outcomes) when providers do not share that information with labs or when labs cannot get it directly from patients.
(See “Lessons Learned From the COVID-19 Pandemic” on p. 42 for more information about how states modernized eCR during the pandemic and are applying the lessons for other diseases.) Worse still, public health officials in many jurisdictions said that cases are underreported overall, with providers more likely to report cases of rare diseases or immediately reportable conditions. As a result, public health agencies rely more on lab reports to create their internal cases, rather than requiring both case and lab reports. However, public health agencies that rely on labs for data may be missing patients who have a reportable illness but did not take a lab test. In addition, lab reports often lack key details (e.g., race and ethnicity, onset date, outcomes) when providers do not share that information with labs or when labs cannot get it directly from patients. Public health officials expressed concern that small and under-resourced providers often cannot afford to transition from manual to eCR-capable systems.
There are challenges with eCR on the receiving end as well. Officials in about half of the jurisdictions Pew interviewed said that, although health departments receive some eCR, they were not incorporating the data into their disease surveillance systems when the research for this report was conducted. A number of these jurisdictions described plans or current activities to use the data and eventually incorporate it into their disease surveillance systems.
Few respondents could specify or even estimate the proportion of case reports they receive electronically. Without this baseline data, health departments cannot know definitively whether their efforts to promote eCR—through requirements, investments, and/or incentives—are having the intended effect.
No jurisdiction requires eCR, but ELR is required in seven. Officials in about two-thirds of jurisdictions said they received 90% or more of their lab reports via ELR. Many respondents reported that timeliness of ELR was generally acceptable. Several jurisdictions reported receiving ELR in near-real time or daily from most labs. Only two reported receiving less than 70% of their lab reports via ELR.
Officials in many jurisdictions described technological challenges with eCR, including duplicate reporting, large file sizes, and lack of storage.
Action items
Jurisdictions should inventory their reporting capacities, statutes, regulations, and agency policies to identify opportunities to improve the quality of case data.
Federal and state policymakers should consider flexible electronic reporting policies for under-resourced or low-volume reporters.
Jurisdictions should build on the progress made during the pandemic to use eCR for COVID and expand it for reporting a broader range of diseases. The CDC reports that eCRs are being used to report a wider range of conditions beyond COVID-19 and is helping facilities implement eCR for more than 170 reportable conditions.37
Complete findings
How do states govern case and lab reporting?
Case and lab reporting is conducted in all 50 states and the District of Columbia, and every jurisdiction has statutes or regulations that require reporting of infectious and environmental diseases of public health concern. It is not optional in any jurisdiction.
Every jurisdiction maintains a list of reportable conditions that must be submitted to the state health department via case reports or lab reports, or both. Forty-five set their lists in formal regulations while others maintain online lists in other published documents.
Almost every jurisdiction—49 for case and 45 for lab—specifies timeliness requirements for reports. In many jurisdictions, those requirements relate to specific conditions (e.g., immediately for anthrax, within 24 hours for measles, within a week for flu, quarterly for methicillin-resistant Staphylococcus aureus).
Almost every jurisdiction—46 for case and 45 for lab—requires certain data elements such as name, address, date of birth, sex, or race and ethnicity in statutes or regulations. (See “Race and Ethnicity Data Across Use Cases” on page 41 for more information.)
Forty-one jurisdictions have both statutes and regulations pertaining to case reporting. The remaining 10 have regulations only. Fifty have regulations related to lab reporting, and 36 also have relevant statutes.
In interviews, officials in 23 jurisdictions said that changing case and lab reporting requirements could be accomplished through the regulatory administrative process. Officials in five indicated that changing regulations in their state requires legislative approval or involvement.
Forty jurisdictions have enforcement mechanisms to penalize reporters for failing to comply with case and lab reporting requirements and related provisions (e.g., privacy and confidentiality). Two have enforcement mechanisms for not complying with case reporting requirements only. Penalties include fines, reports to medical licensing boards, or short-term imprisonment. However, no one we interviewed knew of any instances in which required reporters were penalized for noncompliance.
Respondents from several jurisdictions indicated that compliance with reporting requirements generally relies on strong relationships and communication with reporters. They said that informing health care facilities about data quality or reporting issues often improved the issues without any stronger action.
At least two jurisdictions reportedly work with the Clinical Laboratory Improvement Amendments program to address reporting issues via lab certification inspections and audits.
A few respondents described escalating reporting issues to agency leaders or sending letters to reporters to address reporting problems.
A few respondents indicated that incentives were preferable to penalties.
Who is required to report case and lab data? Providers Every state and the District of Columbia require providers (physicians, nurses, and other clinical care professionals) to submit case reports.
Only two jurisdictions require providers to submit lab reports. Providers are not required to report lab results in the other 49. Laboratories Twenty-one jurisdictions require labs to submit case reports; 30 do not.
All 51 jurisdictions require labs, including those within hospitals, to submit reportable test results. Hospitals Almost all jurisdictions require hospitals to submit case reports, with 45 listing hospitals as required reporters in statutes and regulations. Six do not list hospitals as required case reporters. Other reporters Forty-seven jurisdictions require other individuals and organizations to report case data, including: Schools and school officials in 37.
Child or day care centers in 30.
Dentists in 21.
Correctional facilities in 14.
Pharmacists in 13.
Veterinarians in 10.
Other required reporters include paramedics, emergency medical services, or emergency medical technicians; medical examiners or coroners; local health departments, officers, or boards of health; food or beverage establishments or handlers; and even broad categories using language such as “any persons with knowledge of disease.” States vary in where and how they specify these reporter requirements. For example, states may include paramedics, emergency medical personnel, and medical examiners within their definition of health care providers or list those entities separately from health care providers. States may require groups such as food or beverage establishments to report foodborne outbreaks specifically, while other states may require those same groups to report a broader set of illnesses
How are case and lab data reported?
Officials described how enabling and encouraging more automated electronic reporting—for example, through improvements to standards and EHR systems, and by offering financial incentives through Medicare—would more effectively improve reporting.
In policy Automated electronic reporting Of the four data sources covered in this research, eCR is in the earliest stages of widespread adoption both in policy and in practice. However, automated electronic reporting for lab reports (i.e., ELR) is required in state policy and used in practice most often.
For case reporting, no jurisdiction requires eCR and only two reference automated electronic reporting as a method in statute or regulation; the other 49 jurisdictions do not specifically include or exclude it. Thirty-four also allow electronic methods of reporting more broadly.
In interviews, officials in 15 jurisdictions offered that mandating eCR would not improve reporting within their state, compared with officials in 10 who said it would. Instead of requirements, officials described how enabling and encouraging more automated electronic reporting—for example, through improvements to standards and EHR systems, and by offering financial incentives through Medicare—would more effectively improve reporting.
For lab reporting, three states require ELR and seven specify that it is an optional method. Seven jurisdictions require that lab results be reported electronically without specifying automation, while 27 refer to electronic reporting as an option. Twenty-four jurisdictions allow lab reporting via fax (20), web portals (six), and/or email (two). This reflects how ELR has progressed compared with eCR and provides a model for state policymakers and health officials as they look to expand eCR. In interviews, officials in 20 jurisdictions said that mandating ELR would improve reporting within their state, compared with officials in 10 who said it would not. In states that require ELR, officials said it increased the flow of electronic data. One interviewee from a state with large commercial labs described how requirements there prompted those facilities to send ELRs to “a lot of other states as well.” However, other officials were also cautious about broad mandates for all reporters and preferred a tailored mandate that accommodates smaller and rural reporters. Indeed, a few jurisdictions require ELRs only from labs that exceed a certain threshold, such as 30 reports a month in one state. As one official said, “In order to have a provider or an organization to report electronically, they first need to have the capacity. And if we make that regulation or make that rule [requiring ELR], what will happen to the small entity that doesn’t have the capacity to do so?”
Web portal Web portals are an option for reporting case data in three jurisdictions. They are not specified in the other 48.
Six jurisdictions, including the three that allow web portals for case reporting, specify that labs can report via web portal. Email Reporters can use email to report case data in three jurisdictions and lab results in two. The remaining jurisdictions do not specify. Fax Twenty-five jurisdictions allow case data to be reported via fax; 20 accept lab results via fax. The rest do not specify Mail Mail is an option for reporting case data in 19 jurisdictions and for reporting lab results in 16. The rest do not specify. Telephone Forty-six jurisdictions list telephone as a method for case reporting; 38 list it as a method for labs reporting. The rest did not specify.
Jurisdictions also require providers and labs to report suspicions of certain diseases (e.g., anthrax, measles, tuberculosis) to their state health department by phone within 24 hours. Confirmed cases of other diseases may be required to be reported within 24 hours or other time frames, but not necessarily by phone.
Under-Resourced Providers Benefit From Flexible Data-Reporting Requirements Certified EHR systems that enable eCR and ELR are complicated and expensive to purchase, install, and maintain. Some health care providers can invest in this technology and the workforce to manage it more easily than others. As one official said, “Smaller providers do not have the capacity for HL7 reporting or for establishing electronic case reporting … so we try to meet them in the middle. … We could accept, for example, comma delimited files [such as .CSV spreadsheets] or we set up a system where they could just enter the data directly to the system and it comes directly to us. We try to find ways to make it easier for them.” Another official voiced a concern shared by others that rigid requirements could shut out some providers: “The concern with … mandating [ELR] from the smaller reference labs would be that we would essentially not get that data.” At the same time, health departments may lack the infrastructure to securely receive, process, and store electronic data from every reporter in their state. As a result, taking a stepwise approach to increase capacity gradually, as budgets and workforce allow, may be the most pragmatic course. During the COVID-19 pandemic, the Texas Department of State Health Services started receiving a significant number of lab reports via fax, which normally requires staff to manually process and input the data from paper into databases.38 This was common across the country during the pandemic, as new, temporary facilities, such as pop-up sites, were reporting results but lacked the IT infrastructure of conventional labs to accommodate ELR.39 To meet reporters where they were, the agency developed an “eFaxing” system that automatically converted faxes into data that could go into the disease surveillance system. This ensured that every lab could report test results while also reducing the burden on public health agencies to manually review and input data from paper-based faxes. These intermediate improvements in reporting are valuable in special situations, but because eFaxing still requires labs to take additional administrative steps, facilities that report regularly at higher volumes would still benefit more from the faster, more automated data transmission that ELR offers.
In practice
Although statutes and regulations require providers to report case data for all reportable diseases, many interviewees said that providers substantially underreport cases and are likely to report only cases of rare diseases or immediately reportable conditions. When public health agencies do receive case reports, providers commonly wait until after they get positive results from the lab to share case data, unless they are otherwise required to report when they reasonably suspect a patient has a reportable disease. As a result, case reports often arrive after lab results and with no new information. So, officials said that lab reports were their primary source of initial information about cases of disease.
Because eCR is new in most states and providers report case data inconsistently by any means, few interview respondents were able to estimate the proportion of case reports they receive electronically. However, respondents said fax and telephone were the most common methods for case reporting, followed by web entry, and, rarely, email or paper mail.
Officials in almost all of the jurisdictions Pew interviewed said that they receive some data via eCR, but many do not fully use it. As a result, they are likely missing opportunities to use that data to improve disease surveillance, prevention, and control.
At the time of these interviews, about half of the officials said they were not incorporating eCR data into their surveillance systems. Only one-third said they bring eCR data into their disease surveillance systems and process that data for epidemiology programs to use for analysis. Expanding the use of eCR data into surveillance systems across all conditions requires significant investments of time and resources.
Officials in several jurisdictions said they stored eCR data outside of their surveillance systems, such as in other databases, where staff epidemiologists can access the data if needed to fill additional information (e.g., patient demographics) to advance case investigations.
Of the jurisdictions whose officials said they received eCRs for specific conditions, all received COVID-19 reports. Many also received eCRs for mpox, a viral infection that the World Health Organization declared a public health emergency of international concern in July 2022. 40 A few jurisdictions received eCRs for additional conditions, but no jurisdiction was receiving eCRs for all reportable conditions when this research was conducted.
A few jurisdictions received eCRs for additional conditions, but no jurisdiction was receiving eCRs for all reportable conditions when this research was conducted. Several jurisdictions described the status of their eCR implementation as a test and validation or “parallel production” phase. They continue to use legacy reporting systems while they receive data via eCR in a test environment, evaluate data quality, and work out how that data might be integrated with existing case information.
Several jurisdictions said their surveillance systems were simply not technologically capable of receiving and processing eCR data. Some are waiting to implement new systems rather than incorporate eCR capabilities into their existing platforms.
By contrast, officials in every jurisdiction interviewed reported receiving and using ELRs frequently, whether jurisdictions required it or not.
Officials representing about two-thirds of the jurisdictions Pew interviewed—with and without ELR requirements—estimated receiving 90% or more of their laboratory reports via ELR.
Ten jurisdictions estimated receiving between 70% and less than 90% via ELR.
Two estimated receiving less than 70% via ELR.
National Platforms Facilitate Sharing of Case Reports Across State Lines Because case reporting requirements differ from state to state, health care providers who treat patients from multiple states—for instance, a doctor in a tristate area—may be required to comply with multiple policies using different mechanisms to report different diseases under different timelines to different health departments. To help providers overcome these challenges, the CDC has partnered with the Association of Public Health Laboratories (APHL) and the Council of State and Territorial Epidemiologists to establish two platforms—the APHL Informatics Messaging Services (AIMS) and Reportable Condition Knowledge Management System, which together route data to the appropriate jurisdiction.41 This relieves providers of the burden of navigating multiple jurisdictions’ policies and ensures that those jurisdictions receive the data they need to detect, prevent, and control disease. Many officials reported to Pew that they rely on AIMS’ centralized eCR reporting infrastructure.
How does data flow to public health agencies?
Many jurisdictions require case and lab reports to go straight to their state health department. Many other jurisdictions require case and lab reports to go to local health departments first, which then submit them to the state health department. Two jurisdictions allow case and lab reports to go to local and state health departments concurrently. In some jurisdictions, providers can report to state or local agencies.
Many jurisdictions that described their reporting structure said regardless of whether the eCR and ELR must go to state or local health departments first, they can be made available to both at the same time. With simultaneous access for state and local public health entities, health care providers can fulfill their legal requirement to report diseases to either jurisdiction by sending data to the centralized system. Several interview respondents also noted that, although eCRs were submitted to state agencies, manual reports were more likely to go to local health departments first, especially where local health departments play a key role in case investigation and might have strong relationships with local providers. One benefit of automated electronic systems is that they can transmit data to state and local departments simultaneously without increasing the burden on health care providers; this ensures that agencies at both levels have timelier, more complete, and more accurate data to better detect, prevent, and control diseases in their communities.
In many jurisdictions, reporters can connect to a health information exchange (HIE) to facilitate case and lab reporting. Compared with eCR, this practice is more common for lab reports, which is consistent with their higher proportion of ELR reporting.
What data quality issues challenge health departments?
Interview respondents described a variety of issues that undermine the quality of electronic case and lab data they receive and their ability to analyze and use it.
eCR Duplicate and erroneous reports are often triggered by outdated information in patients’ history. One official estimated that up to 40% of their state’s eCR data were duplicate records. Erroneous reports might be triggered by updates or changes to the health record that prompt EHR systems to automatically generate new reports for the same illness. As one official described it, “The disease of interest was diagnosed four years ago and yet it’s still [marked as] an active problem in the person’s health record. … And you’re like, whoa I need to look at this. And then you spend time looking through this like massive PDF that they sent you and you realize, oh, they had TB in 1998. Great. … And then they come back in for a recheck and you see their chart again because, guess what, their TB from 1998 is still showing up.” Officials also noted that EHRs may send patient information unrelated to the reportable condition, raising privacy concerns. Because eCRs are large files, duplicate and erroneous reports increase data storage costs, which can be prohibitive for under-resourced health departments. Respondents noted that a lack of national standards often drives data quality issues. This is consistent with recommendations from numerous entities working on data modernization at the national level, such as the Health Information Technology Advisory Committee and the CDC Advisory Committee to the Director Data and Surveillance Workgroup. 42
ELR Many respondents reported issues with completeness and accuracy of key data elements, including patient contact information, addresses, race, and ethnicity, and identified these elements as active areas for improvement. Patients’ phone numbers and addresses are critical for contact tracing or further investigation and for accurately determining jurisdiction, which is usually based on where a patient lives. Several respondents also reported issues with appropriate use of standardized codes for reporting lab tests and results. These codes are key for identifying the disease or condition the report is about, determining the geographic location of outbreaks or clusters, accurately and automatically processing reports into state surveillance systems, and ensuring the data can be analyzed and used by epidemiologists. Many respondents reported that timeliness of ELR was generally acceptable. Several jurisdictions reported receiving ELR in near-real time or daily from most labs.
During the COVID-19 pandemic, Michigan’s Department of Health and Human Services observed that lab reports were often missing race and ethnicity data and other key details necessary to track the disease in different communities and populations.43 By publishing report cards that compared the completeness of lab reports, the agency stoked “friendly competition” and incentivized labs to submit more complete reports and freed up staff to focus on core public health functions rather than assessing and improving data quality.
What are states doing to ensure data quality for eCR and ELR?
Existing messaging standards
Many interviewees reported using the HL7 standard for eCR, known as eICR (electronic initial case report).
For ELR, most respondents reported using HL7 standards, with many specifically citing the HL7 Version 2.5.1 Implementation Guide for ELR. Several said they also accepted the older 2.3.1 standard, converting to the current version in their own systems.
For ELR, respondents also reported different approaches to promoting standards. Several jurisdictions described their approach as strict, adhering closely to the requirements or applying additional requirements beyond the national HL7 implementation guide. The same number of jurisdictions described their approach as more permissive, enforcing fewer requirements than the national standard. Interviewees described a tension between strict adherence to the standard to prevent data quality issues downstream and being permissive to avoid missing reports and discouraging reporting One example of different approaches relates to the use of standard terminology for lab tests and results. The HL7 implementation guide calls for the use of two common sets of codes to standardize the way specific lab tests and results are reported. Labs, however, often use their own local codes in their systems, which they must manually translate to those common codes before reporting them to their health departments. A few officials said that their states strictly require labs to adhere to those common codes, whereas officials in two jurisdictions said health agencies accepted local lab codes and performed the manual translation themselves.
Onboarding new reporters
For eCR and ELR, the process of onboarding new reporters is a critical point for testing initial connections between providers’ and state health departments’ systems, assessing data quality, and preventing issues once reporting goes live.
Many jurisdictions reported that the CDC onboarding team handled setup for connections to AIMS for eCR as well as initial validation. A few jurisdictions described performing additional validation for reports after onboarding
For ELR, many jurisdictions described extensive validation checks and testing during onboarding to ensure that messages adhere to the HL7 standard and key data elements are populated correctly.
At least two respondents reported having disease domain experts check the data to ensure accuracy and usability.
A few jurisdictions described a period of parallel production after going live. If they find that a reporter’s manual data matches its ELR data, the lab can stop sending manual reports and rely solely on ELR
Monitoring data quality
Interview respondents described different approaches to monitoring ongoing data quality.
Data quality practices for ELR were well established. Many respondents reported using routine validation checks, often automated, to ensure that incoming messages conform to HL7 standards and that essential data elements are complete before incorporating data into their surveillance systems.
Several jurisdictions described manual elements to ELR data quality processes, particularly for handling errors identified by validation checks. Staff review messages that fail these checks and can manually correct them or follow up with labs to correct them. Reports submitted in a .CSV format often require more manual review and cleaning than HL7 messages.
Many respondents reported monitoring ELR data feeds for reporting volumes and frequencies, looking for any drops or changes in established patterns according to reporting facilities and reportable conditions. Several of these jurisdictions also reported using dashboards or reports to assist with this monitoring and notify staff about changes.
Several respondents said that their jurisdictions are still working out data quality processes for eCR. Officials also described data quality processes for eCR that were similar to those used for ELR, including validation checks for conformance to standards and completeness of critical data elements and monitoring of reporting volumes.
Improving data quality
Most respondents described working with providers and facilities to improve data quality, stressing the importance of communication and strong relationships with partners to facilitate better reporting.
Officials in several jurisdictions said they provided feedback to reporters on their own data quality via report cards or dashboards, comparing their performance with other facilities or state averages.
A few respondents said they tried to fill missing data elements (e.g., contact information, race) by matching to other available data sources, such as an HIE with patient demographic data, state drivers’ license databases, and online address verification services.
States are working to improve automated integration of ELR into disease surveillance systems through technical workflows and validation rules. Although states manage most infectious disease data in integrated disease surveillance systems, some also have separate, siloed systems for specific conditions, such as sexually transmitted infections, that may require manual steps to incorporate ELR data.
Syndromic surveillance
Syndromic surveillance is designed to be an early-warning system for urgent threats. It received renewed attention in the wake of the 9/11 and anthrax attacks of 2001.44 Coinciding with the development of electronic data systems, syndromic surveillance benefits from automated, real-time data reporting with little to no work required from health care providers once they connect to the system.
Local, state, or national syndromic surveillance systems collect patient data when providers—mostly doctors and nurses in emergency departments* —see, admit, transfer, or discharge patients.45 The information is anonymized and includes some combination of patients’ chief complaints, symptoms (e.g., sore throat), signs (e.g., temperature), diagnoses, demographic details, and location.46
The system is designed for speed. It can often take days or weeks to test and diagnose a patient, and longer still for the related case and lab data to reach a public health agency. By comparison, because syndromic data is based on symptoms and signs that define a syndrome (e.g., influenza-like illness), data can be generated and reported more quickly than other types of public health reporting.47
State and local health departments expanded their syndromic surveillance capacity after the CDC began redesigning its BioSense program in 2010.48 BioSense has since evolved into the National Syndromic Surveillance Program (NSSP). Many jurisdictions use the analytic tools and services within NSSP’s cloud-based BioSense Platform to track seasonal influenza-like illnesses and were able to build on this experience to provide critical information about COVID-19 symptoms during the pandemic.49 States have used the data for a wide range of purposes, including to improve support for people experiencing homelessness in the state of Washington, to understand and mitigate the impact of wildfire smoke on the public’s health in Oregon, and manage a dengue outbreak in Florida with smarter mosquito-control strategies.50
By monitoring for spikes in syndromes or other trends, officials have been able to identify and respond to threats quickly and develop targeted interventions and messaging to address the health conditions facing their communities (e.g., wildfire smoke, exposure to chemical hazards in occupational settings). One official described syndromic surveillance as “a very robust tool for a lot of nontraditional public health issues—drug overdoses, suicide, heat stroke—things where there’s no case-based reporting. There’s no lab test to do and so it’s getting utilized more and more beyond traditional situational awareness.”
* Syndromic surveillance can also include data from urgent care centers, poison control centers, emergency medical service agencies, and other providers.
Key takeaways
Compared with case, lab, and immunization reporting, syndromic surveillance comes with the fewest reporting requirements; only 13 jurisdictions mandate syndromic surveillance reporting (mostly by emergency departments). This excludes jurisdictions that collect syndromic surveillance data under authorities established through broader statutes or regulations. Participation is higher: Of the jurisdictions whose public health officials Pew interviewed, about two-thirds reported receiving syndromic data from 75% or more of the emergency departments in their state.
Officials in many jurisdictions said that the Centers for Medicare & Medicaid Services’ Promoting Interoperability Program effectively incentivized hospitals to report syndromic surveillance data.
Even though patients are increasingly visiting urgent care clinics instead of emergency departments, only three jurisdictions require these facilities to report syndromic surveillance data.51 Without broader reporting requirements, state health departments may be missing opportunities to detect and respond quickly to emerging threats. Interviewees said that a main barrier to expanding urgent care reporting is the cost to the state for establishing connections and onboarding these facilities.
Action items
States’ public health agencies should measure the participation of urgent care centers in syndromic surveillance reporting and consider—to the extent these places provide a significant and growing proportion of emergency care—whether new policies or initiatives are needed to encourage or require more urgent care centers to report syndromes.
State public health agencies should take an inventory of their reporting capacities, statutes, regulations, and policies to identify opportunities for increasing the reporting among eligible health care facilities where no data has been recently sent to the NSSP BioSense Platform.52
Complete findings
How do states govern syndromic surveillance reporting?
Many respondents found National Syndromic Surveillance Program tools useful and reported reviewing their data summary dashboards to ensure they were receiving sufficiently detailed data from all facilities with which they were connected. However, the frequency with which respondents assess data quality varied dramatically, with some monitoring daily and others quarterly.
While almost all jurisdictions have active statewide syndromic surveillance programs, two state public health agencies interviewed had limited programs, one that covered symptoms for a few conditions only (e.g., COVID-19) and another in which data was reported in only some counties.
Most syndromic surveillance data is provided voluntarily. Only 13 jurisdictions require statewide syndromic surveillance reporting. Three have statutes or regulations that enable their state health department to establish a syndromic surveillance system, but these laws either do not mandate reporting or only specify that the health authority may require reporting. Pew’s count excludes jurisdictions that collect syndromic surveillance data under authorities established through broader statutes or regulations. Four jurisdictions explicitly require providers to report syndromic surveillance data via automated electronic means, while four require generic electronic reporting.
Officials in 10 jurisdictions that did not already require syndromic surveillance reporting said that instituting a reporting requirement would improve reporting within their state, whereas officials in eight believed that it would not, in part because participation was high already and incentivized by CMS’ Promoting Interoperability Program.
In interviews, officials in five jurisdictions said that requiring syndromic surveillance reporting could be accomplished through the regulatory process. Officials in two indicated that they would probably need legislation to require syndromic reporting.
One state includes a list of specific syndromes in regulation. Others provide a list of syndromes on their websites or in nonbinding guidance. Limiting the symptoms, signs, and syndromes that should be reported may prevent public health departments from tracking conditions of interest and identifying unexpected threats.
Five of the 13 jurisdictions that require syndromic surveillance reporting specify data standards in statute or regulation. It is more common for states to communicate required standards via onboarding and implementation guides. States may benefit from the more common approach, which enables public health agencies to adapt to new standards and technologies
Five of the 13 jurisdictions that require syndromic surveillance have an enforcement provision in statute or regulation. However, no one shared instances in which reporters were penalized for not complying with data-reporting requirements.
In practice, state public health officials reported that they receive syndromic surveillance data most often from emergency departments, less often from urgent care centers, and least often from outpatient clinics.
Emergency departments Of the 43 jurisdictions whose public health officials Pew interviewed, about two-thirds reported receiving data from 75% or more of the emergency departments in their state. Only one state official reported receiving less data than that; the remaining responses were not specific enough to further categorize. Several jurisdictions did not specifically comment on the presence of emergency department data, though the majority of these reported receiving hospital data that likely encompassed emergency as well as inpatient data.
Urgent care At the time of these interviews, only one jurisdiction reported receiving data from 75% or more of the available urgent cares. Several jurisdictions reported receiving limited urgent care data. Some had invested in building electronic connections to urgent cares in communities that lack an emergency department or urgent cares affiliated with hospitals or health systems that were also providing emergency department data. Urgent care participation may increase in the coming years, as several jurisdictions reported that they are working to expand relationships with and connections to these facilities. Two jurisdictions, however, explicitly reported difficulty in previous expansion attempts, including legal limits to current authorizations. Officials said cost may be a barrier to getting small and independent urgent care clinics to connect with syndromic surveillance systems. It is important to note that CMS’ Promoting Interoperability Program does not incentivize urgent care centers to report syndromic surveillance data.
Urgent Care Centers Can Be a Vital Source of Syndromic Surveillance Data Urgent care centers could be a growing source of syndromic data. A 2022 literature review conducted by researchers at the University of California-San Francisco found that urgent care visits increased substantially from the late 2000s to the late 2010s and that the increase was associated with a drop in emergency department visits.53 A 2021 study found that “having an open urgent care center in a ZIP code reduced the total number of ED [emergency department] visits by residents in that ZIP code by 17.2%.”54 If states have limited authority to compel urgent care facilities to provide this data, they may miss opportunities to detect emerging threats and estimate the burden of illnesses.
Outpatient clinics Several jurisdictions reported receiving data from a subset of outpatient clinics, particularly those that are part of or affiliated with hospitals or health systems. One agency reported that it lacked authorization to require outpatient clinics to report syndromic surveillance data.
How does data flow from reporters to public health?
Syndromic surveillance data usually ends up in the NSSP BioSense Platform and with state health departments, but the data takes different paths depending on how states govern the reporting process.
Of the 13 jurisdictions that require syndromic surveillance reporting, about half use their own platform, while in the other half, reporters submit data directly to the NSSP BioSense Platform, which states can then access and analyze.
Officials in 28 jurisdictions, including those with and without reporting requirements, described their respective state’s NSSP BioSense reporting structure. Consistent with the legal analysis, slightly more than half said they collect and manage syndromic surveillance data before routing it to the platform, while the others reported that they established direct connections to that platform.
States Can Benefit if They Collect Syndromic Surveillance Data Directly From Hospitals States that rely on the data reported directly to the NSSP BioSense Platform may have access to less data than states that use their own platform and can require or encourage the reporting of data elements that are relevant to each jurisdiction’s particular health issues. Officials from one state agency noted that collecting data directly from providers rather than from BioSense helped them tackle specific health issues that required more detailed data than what the platform could provide, including special studies related to injury and violence prevention programs.
Facilities may report data first to a HIE, a third-party platform that serves as an electronic hub for different organizations to share data, including with the NSSP BioSense Platform. States employ HIEs in a variety of ways: for example, to supplement data that public health agencies need from health care providers, send clinical information securely across providers who see the same patients (such as primary care providers and specialists), or enable an emergency department to quickly access patient medical records. 55 Respondents in many jurisdictions said that HIEs supported reporting, including several that did so for a substantial proportion of data. Several jurisdictions explicitly reported an absence of HIE involvement in syndromic surveillance.
How do states ensure data quality?
Respondents described a variety of methods to monitor and improve the quality of syndromic surveillance data.
Data quality efforts typically begin with the onboarding process, when health departments help health care providers connect their EHR platform to the state’s syndromic surveillance system and ensure that the automated messages are coming through consistently, quickly, and accurately. As one respondent noted, establishing syndromic surveillance connections is the first opportunity for health care facilities to demonstrate that their data conforms to technological and formatting standards. Seven jurisdictions identified challenges related to onboarding, noting that the process is often lengthy and time-consuming, and can be burdensome for both the department and the provider or facility reporting data, that switching vendors sometimes complicates the process, and that the processes and standards required are not always clear and can be difficult to communicate to providers. As one official said, onboarding “can take about one to two years sometimes. So, it would be nice if you could have some kind of requirement or … time constraint about how long an onboarding process or vendor switch can take, because a lot of times during these vendor switches, we will experience the syndromic outage [a period during which a facility is not reporting syndromic data]. So, we don’t have complete coverage over all of our counties right now or all of our facility region.” Another official said onboarding required more funding: “With COVID, [funding] got better but prior to COVID it was very minimal. … It takes a lot of training and time to get someone to the point where they can even be comfortable doing these sorts of activities.” To support participation in the NSSP, state agencies rely on national messaging guidelines developed by the CDC’s Public Health Information Network (PHIN). 56 Used during onboarding, this PHIN messaging guide incorporates standard HL7 message formats. A few respondents acknowledged receiving syndromic surveillance data in an older version of the HL7 standard or otherwise not adhering as strictly to the standard. For example, one respondent described giving less experienced facilities “leeway because, as the state, we have to collect the data.” As the respondent explained, they “accept some data that is not up to par, per se” while they work with facilities to upgrade and improve their submissions.
After onboarding, state agencies use a mix of ad hoc and systematic methods to ensure that facilities continue to report data of sufficient quality. For example, one respondent mentioned reaching out “if a facility stops sending a variable that I find useful” so the facility knows it needs to reconnect and send that data. Another respondent described an automated method using a computer program to make sure required data is present. Many respondents found NSSP tools useful and reported reviewing their data summary dashboards to ensure they were receiving sufficiently detailed data from all facilities with which they were connected. However, the frequency with which respondents assess data quality varied dramatically, with some monitoring daily and others quarterly. Respondents also reported that they use dashboards from HIEs and other vendors involved in data transmission to assess data quality. One respondent felt confident that their general monitoring could ensure the timeliness of everything that arrived and the inclusion of the chief complaint but did not think the state agency had sufficient insight into information that might be missing. As this respondent explained, “If they don’t send it, we don’t know.” Another respondent said staff were so focused on onboarding new facilities that they did not have time to work with existing reporters to improve data quality.
Immunization reporting
After a measles outbreak claimed dozens of children’s lives and sickened more than 27,600 others from 1989 to 1991, public health leaders concluded that more illnesses might have been prevented if doctors could have more easily determined when their patients were missing vaccinations.57 Within a few years, state and local health departments began establishing Immunization Information Systems (IIS), originally termed immunization registries, to confidentially and electronically record vaccinations administered by health care providers within their jurisdiction.58
In addition to helping providers identify which vaccines their patients have received and still need, the data also helps public health officials identify communities and populations with inadequate vaccine access, determine if vaccination rates for certain diseases are low, and plan interventions and allocate resources to increase immunizations. By the time of the COVID-19 vaccine, many IIS enabled providers and public health officials to manage vaccine administration and supply.59
These systems are now used in every U.S. state and territory and some cities. The CDC required providers participating in the agency’s COVID-19 vaccination program to report the vaccinations to their jurisdiction’s IIS or another CDC-designated system.60 Under normal circumstances and for all other vaccinations, each IIS operates according to a unique set of its jurisdiction’s policies, differing in terms of whether reporting is required or voluntary, who must report, which vaccinations they must report and what details they must include, how quickly and by what method they must report, and how health departments manage the immunization data and ensure its quality, among other variables.
Key takeaways
Officials in almost every state estimated that most immunizations are electronically reported—50% to 95% via automated electronic systems and 8% to 50% via web portals. Unlike case reports, a small portion of data is transmitted via other, more labor-intensive means such as fax. This tracks with policy: State statutes and regulations most commonly cite electronic reporting as an acceptable method for submitting immunization data. In addition, several jurisdictions reference automated electronic reporting and web portals as acceptable reporting methods. However, automated electronic reporting is optional and not required by statute or regulation.
Policies may not reflect the full range of vaccination providers or vaccinated individuals. However, practices focused on enabling or encouraging electronic immunization reporting and data exchange across states, along with decades of efforts among practitioners to standardize IIS reporting, have contributed to high percentages of children, adolescents, and adults whose data is in their jurisdiction’s IIS. 61
The HL7 standard is designed to facilitate data reporting, but its implementation can affect data quality. As a result, respondents had mixed opinions about its impact. Roughly a third of the officials who expressed an opinion regarding HL7 said it produced better data, a third said it undermined data quality, and a third said it had no impact.
Action items
Because immunization data comes from multiple sources, jurisdictions with narrow reporting requirements, such as those that specify certain types of health care providers and settings or reporting for some ages, may not capture all immunizations being administered in the state, despite robust reporting in their IIS. Jurisdictions should examine how their immunization reporting requirements and activities to improve electronic reporting align with where and from whom people are receiving vaccinations. For example, if schools and workplaces are administering a significant proportion of vaccines but represent a smaller proportion of electronic reports, jurisdictions could consider what balance of policies and practices can most effectively increase the quantity and improve the quality of immunization data they receive.
Jurisdictions should assess the extent to which vaccine providers are using manual tools to report immunizations and identify opportunities to help providers—particularly low-volume and under-resourced providers—go digital.
Complete findings
How do states govern immunization reporting?
Immunization reporting is required in some form in 45 jurisdictions and voluntary in three. Seven jurisdictions specify automated electronic reporting as an option, but none requires reporting via that method. Five require reporting via generic electronic methods. While almost all jurisdictions enable adults to determine whether their data is reported to the IIS—either by allowing them to opt out of data sharing or by requiring them to explicitly opt in—several mandate reporting without giving adults the option.
Of the 45 jurisdictions with immunization reporting requirements, 34 have requirements for all ages; 11 require reporting for children’s immunizations alone. States that require reporting for children’s immunizations alone vary in how they define ages of children. In interviews, a few respondents identified newborns as a particularly difficult population in which to track vaccinations because the child might not have a name when vaccines are administered (e.g., when newborns are immunized against hepatitis B). Hospital staff sometimes enter generic names (for example, “Baby boy”) into the hospital record and never update the record to reflect the child’s given name before the EHR closes the health encounter and submits the vaccination record to the IIS. Because these records are designed to help patients and providers keep track of immunizations, the records have little to no value if they lack a valid or correct name.
Several jurisdictions have requirements for specific immunizations. Examples of this include reporting requirements specifically for COVID-19 vaccines, publicly funded vaccines, or vaccines administered during a public health emergency.
Nearly every state has statutes or regulations that address immunization reporting; 30 address it in statutes and regulations, 13 in statute only, and five by regulation only. Only three have neither. In interviews, officials in 13 jurisdictions said that changing immunization policies would require legislative approval or involvement to change statutes. Officials in 10 jurisdictions said they could shift policy through the regulatory process, though two indicated that their states’ regulatory process would require legislative approval or involvement.
In almost all jurisdictions, immunization data is reported straight to the state department of health. One state allows immunization data to be reported to local health departments concurrently with the state department, and a few jurisdictions have unclear reporting structures.
Forty jurisdictions have immunization statutes and regulations that specify confidentiality or privacy of data shared, though jurisdictions also have policies that apply to medical and public health data broadly, along with laws governing privacy and confidentiality that may affect immunization data exchange. Seven do not list policies that address the confidentiality of immunization data.
Eighteen jurisdictions have enforcement mechanisms for providers who are not in compliance with reporting requirements. These most often consist of fines and fees, with some jurisdictions specifying that noncompliance could result in a misdemeanor. No one Pew interviewed reported issuing fines or other penalties.
Of the officials in 41 jurisdictions that participated in interviews about immunizations, 35 confirmed that doctors can query their IIS to see patients’ history of vaccinations, but providers cannot do so in six jurisdictions. … If doctors can only submit immunization data to an IIS but cannot query the system for their patients’ vaccine records, then they cannot identify which of their patients need vaccines—a core problem that IIS were designed to solve.
How is immunization data reported?
Fourteen jurisdictions’ statutes or regulations refer to electronic reporting as an acceptable method for submitting immunization data, and five require it. Seven other jurisdictions describe automated electronic methods as an option for reporting in statute or regulation. As previously stated, none requires it. Electronic reporting methods are not mentioned at all in 24. Officials in 15 jurisdictions said that requiring electronic reporting for immunizations would not improve data within their state. Officials in five believed that it would. Officials who did not want requirements pointed to capacity issues for small and rural providers. As one said, “We have a couple of rurally located public health facilities that serve a very small population of patients, and they only give a few vaccinations a month. And for them to purchase an EHR that would allow them to do electronic documentation and electronic data reporting really just … doesn’t make sense for their facility.”
Seven jurisdictions cite web portals as an option for reporting. These systems require manual data entry by the reporting vaccine provider
One jurisdiction’s statute refers to mail as an acceptable method of reporting immunization data, one state refers to fax, one refers to telephone, and none refers to email.
Twenty-two jurisdictions do not specify any method of transmission. This includes jurisdictions where reporting is required and where it is voluntary
In practice, officials in almost every jurisdiction shared that they most often received data via automated electronic reports and other electronic reporting methods such as web portals. They estimated that: Automated electronic reports accounted for about 50% to 95% of all immunization submissions from health care providers. Web portals accounted for an estimated 8% to 50% of all immunization received from providers. Faxes, spreadsheets, and batch uploads made up 5% or less of the total volume of immunization submissions for any one state’s IIS.
Many jurisdictions specify in guidance that health departments can receive immunization data via an HIE. Three jurisdictions specify that in statute or regulation. Other jurisdictions do not specify that HIEs can play this role but also do not disallow it. In practice, the officials Pew interviewed said that reporters often use HIEs, though responses ranged from estimating less than 5% of all electronic submissions in one jurisdiction to 100% in another. In interviews, officials from several jurisdictions stated that the HIE only transported the message. In other jurisdictions, however, HIEs may store the data, giving public health agencies access to technology tools readily available in an HIE, such as business intelligence software programs to help analyze or visualize immunization data. Although their capabilities differ, during the pandemic, some HIEs augmented the data by using their master patient indexes (comprehensive, accurate lists of people who receive health care services in their geographic areas) to prevent duplicate records in the IIS and to add new data elements, such as race and ethnicity, to the immunization record. 62
Respondents from almost all jurisdictions reported using HL7 as their electronic messaging standard. As several respondents reported, this allowed them to use an HL7 implementation guide to maintain quality by setting
Investments in Rapid Outbreak Responses: Spend Now, Save Later
In an outbreak response, speed is life. Global health financing mechanisms need to address the challenges countries face in responding rapidly to health threats. Countries need to be able to identify outbreaks early, report them quicky, and respond rapidly to stop their spread. Most disaster or response funding mechanisms aren’t set up to facilitate taking these steps in the quick succession needed to prevent epidemics, authors say. The time it took to detect the first cases of Ebola and begin effective response efforts shortened significantly, they say. It was possible due to excellent work of response teams supported by the rapid release of emergency funds in DRC. In Nigeria, RTSL partnered with the African Field Epidemiology Network and Nigeria Centre for Disease Control and Prevention (NCDC) to establish an ROF mechanism that allowed for disbursement of funds within 48 hours. This rapid funding enabled the deployment of more than 200. personnel to support emergency operations across the country. Since its start in March 2019, this mechanism has supported several national responses to several outbreaks.
Global health faces a shifting financial development landscape. Delegates at the Group of Twenty session in September 2023 called for significant reform of financial institutions, and 2024 is the year that critical decisions on global health fundraising will be made. Amid those shifts is an opportunity both to improve the way money is disbursed and used on the ground and to fill critical needs in emergency funding. In addition to replenishing funds for global institutions, governments also need to meet their domestic health spending commitments—which includes domestic funding tailored for speed and sustainability.
Having access to flexible funds changes the game for public health officials working on the ground. Typically, they need to make standalone requests for funds before they can deploy response teams; meanwhile, the disease is left to spread unchecked.
Flexible funding allocated in advance is critical to fulfill the urgent, operational support needs common in the early stages of any response: transportation to get boots on the ground, access to internet and cell phone credit, and personal protective equipment to keep workers safe.
Never was the need for rapid, predictable funding more apparent than during outbreaks of Ebola in Democratic Republic of Congo (DRC) between 2018 and 2022. Whereas the 2018 outbreak lasted two years, more recent episodes in 2021 and 2022 were over in a matter of months. What changed? The time it took to detect the first cases of Ebola and begin effective response efforts shortened significantly.
A health worker is seen preparing a facility for Ebola patients at the Ebola treatment center, in North Kivu, Democratic Republic of Congo, on August 18, 2018. REUTERS/Samuel Mambo
During DRC’s 2018 Ebola outbreak, no suspected cases were identified and no alerts were issued for two months, allowing the disease to spread rapidly throughout the country. By contrast, in August 2022, public health responders were able to investigate and respond to the outbreak in just three days. Six weeks later, the outbreak was declared over with only one confirmed case. This successful response was possible due to excellent work of response teams supported by the rapid release of emergency funds.
Stopping health threats before they become deadlier—and costlier—epidemics requires the kind of fast action taken in DRC’s 2022 Ebola response. Countries need to be able to identify outbreaks early, report them quicky, and respond rapidly to stop their spread. Unfortunately, most disaster or response funding mechanisms aren’t set up to facilitate taking these steps in the quick succession needed to prevent epidemics.
Pilots of rapid outbreak financing (ROF) mechanisms conducted by Resolve to Save Lives (RTSL) have shown that these funds don’t need to be substantial. Resolving global health issues often costs millions—but RTSL has found that spending $5,000 at the first sign of an outbreak can eliminate the need to spend tens or hundreds of thousands to control an epidemic later, or billions to address far-reaching social and economic impacts. Having fast access to funds that allow for flexible spending at the start of an outbreak is critical.
A Field Test
Although countries have improved their ability to detect more outbreaks earlier, funding constraints often prevent them from moving quickly beyond initial identification and reporting to mounting a full investigation, verification, and response.
We tested the ROF approach with our partners in several African countries and found that it not only filled a critical gap in emergency funding, but also significantly reduced the time from outbreak detection to verification and response.
Countries need to be able to identify outbreaks early, report them quicky, and respond rapidly to stop their spread
In Nigeria, RTSL partnered with the African Field Epidemiology Network and Nigeria Centre for Disease Control and Prevention (NCDC) to establish an ROF mechanism that allowed for disbursement of funds within 48 hours of request. When COVID-19 hit, NCDC was able to use ROF to begin preparedness and response activities before the disease was officially declared a pandemic. This rapid funding enabled the deployment of more than 200 personnel to initiate response efforts in 34 of Nigeria’s 36 states and support emergency operations and disease surveillance across the country.
But those successes went beyond COVID-19, equipping NCDC with the resources to respond to 15 additional outbreaks in 2019 and 2020. During a measles outbreak in June 2019, the NCDC was able to deploy a rapid response team in less than 72 hours to investigate and track cases. Just three months later, the outbreak ended with 275 suspected cases and 18 deaths among confirmed cases—much less than in previous outbreaks. Since its start in March 2019, this funding mechanism also supported national responses to several outbreaks of mpox, measles, yellow fever, and Lassa fever.
Implementing ROF has shown significant reduction in response times—from an average of six days to just two in Nigeria—and a subsequent rapid containment of deadly diseases.
The world can take lessons from Nigeria, which was able to leverage the successes of ROF to secure additional funds and expand the availability of ROF to the subnational level as well as start the transition to government ownership and financing. Because most disease outbreaks are identified by clinicians working with communities, availability of rapid funding at local levels will further strengthen the ability to find and respond to new health threats.
An essential element of pandemic preparedness planning is giving countries the tools they need to respond to events before they become outbreaks. Communities should push governments, multilateral development banks, and global health organizations to make fast, flexible funds available to investigate and prevent outbreaks. It could require new mechanisms that balance speed and financial accountability, but our organization has shown that it is possible—and critical for saving lives.
A health worker collects a nose swab from a man at a drive-through sample collection center for COVID-19, in Abuja, Nigeria, on January 14, 2021. REUTERS/Afolabi Sotunde
Amanda McClelland, a registered nurse, is the senior vice president of Prevent Epidemics at Resolve to Save Lives, where she leads a global team working to accelerate progress to make the world safer from the next epidemic.
Coronavirus Outbreak Updates: WHO reviews impact on operations caused by US funding withdrawal, says will work with partners to fill financial gaps
Donald Trump halts World Health Organization funding. He said the group had promoted China’s “disinformation” about the virus. The United States is the biggest overall donor to the Geneva-based WHO.
Coronavirus in US Latest Update
Donald Trump halts World Health Organization funding
President Donald Trump said on Tuesday he would halt funding to the World Health Organization over its handling of the coronavirus pandemic while his administration reviews its response to the global crisis.
Trump, at a White House news conference, said the WHO had “failed in its basic duty and it must be held accountable.” He said the group had promoted China’s “disinformation” about the virus that likely led to a wider outbreak of the virus than otherwise would have occurred.
The United States is the biggest overall donor to the Geneva-based WHO, contributing more than $400 million in 2019, roughly 15% of its budget.
The hold on funding was expected. Trump has been increasingly critical of the organization as the global health crisis has continued, and he has reacted angrily to criticism of his administration’s response.
